Korean J Transplant.
2023 Sep;37(3):189-196. 10.4285/kjt.23.0033.
The role of acidification of the medium on the erythrocyte agglutinating and adsorbing properties of blood group-specific monoclonal antibodies
- Affiliations
-
- 1Sytenko Institute of Spine and Joint Pathology, National Academy of Medical Sciences of Ukraine, Kharkiv, Ukraine
- 2Ukrainian Scientific Pharmacopeial Center for the Quality of Medicines, Kharkiv, Ukraine
- KMID: 2546485
- DOI: http://doi.org/10.4285/kjt.23.0033
Abstract
- Background
Studies on the specificity of ABH antigen-antibody interactions at different pH values are rare. Therefore, the aim of this study was to estimate the effect of acidification of the reacting medium on the agglutinating ability of anti-A monoclonal antibodies (mABs) and their inhibition by glycoconjugates of red blood cell membranes.
Methods
Anti-A mABs were obtained from the Fourth International Workshop on Monoclonal Antibodies Against Human Red Blood Cell and Related Antigens (on July 19-20, 2002, in Paris, France). The glycoconjugates were isolated from erythrocytes' membranes. The inhibition of the lipid and protein isotypes of the blood group A antigen was assessed.
Results
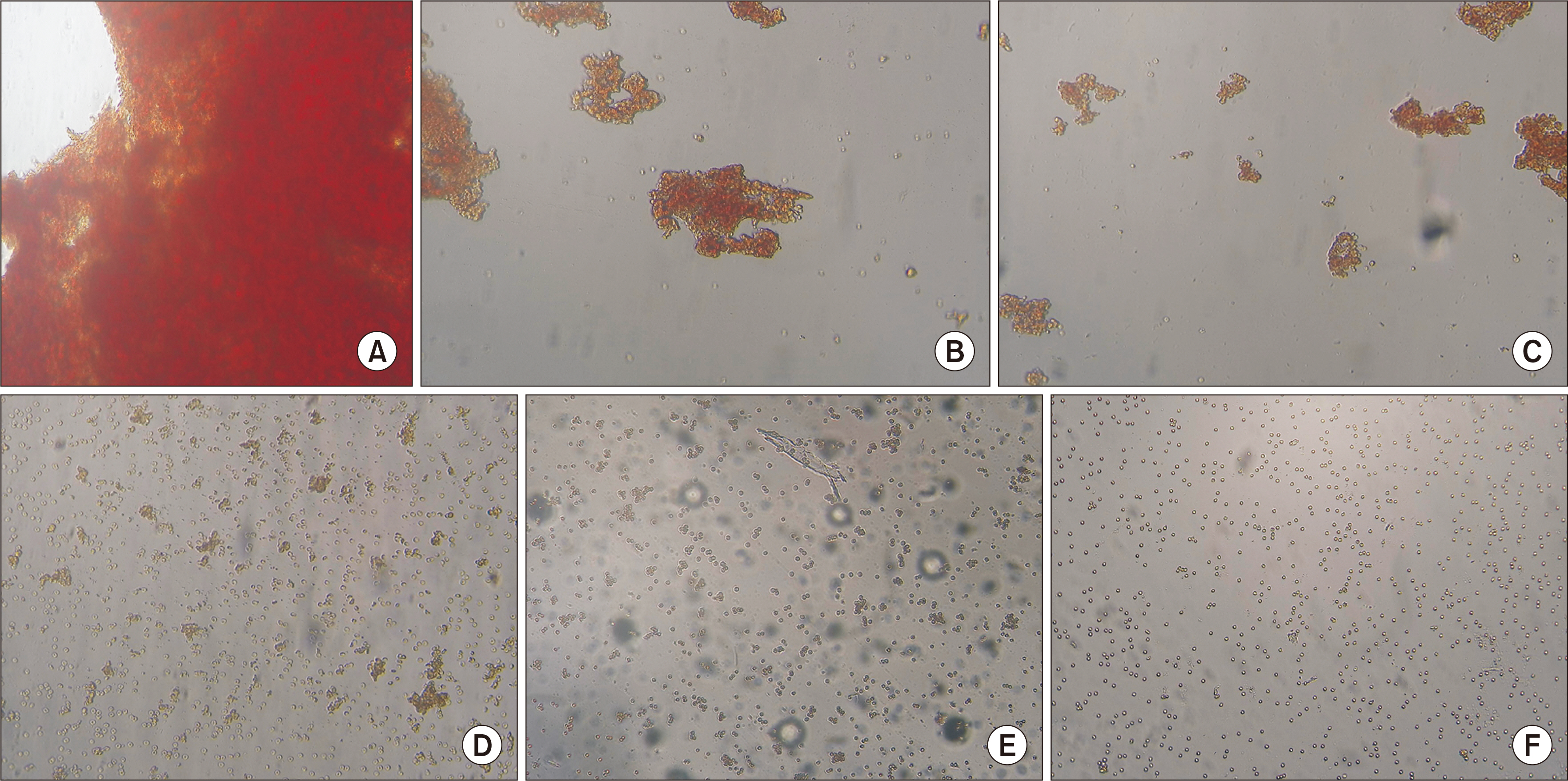
The acidic medium decreased the agglutinating ability of acid immunoglobulin M (IgM) anti-A mABs, in contrast to alkaline immunoglobulin G (IgG) mABs. Meanwhile, at pH 6.5, IgM antibodies exhibited a high adsorption capacity, while IgG antibodies demonstrated a strong adsorption capacity at an alkaline pH. mABs 2-19, 2-28, 2-22, and 2-8 were inhibited by the acidic lipid and protein glycotopes of erythrocyte membranes. However, mAB 2-8 was inhibited by both acidic and alkaline glycotope variants.
Conclusions
The agglutinating and adsorbing abilities of mABs, which are revealed at opposing pH values, should be taken into account during studies of antigen-antibody interactions.
Keyword
Figure
Reference
-
1. Boyd WC. Fundamentals of immunology. 2nd ed. Interscience Publishers;1947.2. Brockhaus M. Immunological research methods. Academic Press;1988. p. 160–2.3. Delevsky YP, Gavrilova LV, Khavkina LV. 1980; Indicators of cellular immunity in its organ-specific manifestations in spinal osteochondrosis. Modern methods of treatment of degenerative-destructive diseases. Medicine. 1:S108111.4. Delevskiĭ IP. Study of the nature of A1- and A2-antigenic differences with use of monoclonal antibodies: a role of glycoprotein and glycolipid epitopes in their formation. Klin Lab Diagn. 2006; (10):6–11. PMID: 17144537.5. Proceedings of the Fourth International Workshop on Monoclonal Antibodies Against Human Red Blood Cell and Related Antigens. Under the Aegis of the Institut National de la Transfusion Saguine and of the Etablissement Francais du Sang. July 19-20. Paris, France. Transfus Clin Biol. 2002; 9:1–108. PMID: 11958157.6. Conrath TB. Handbook of microtiter procedures. Dynatech Corp;1972.7. Dausset J. 1953; Immunohematology of platelets and leukocytes. Presse Med (1893). 61:1533–5. PMID: 13134017.8. Delevskiĭ IP, Zinchenko AA. Comparison of the antigenic spectrum A1, A2, and Ax erythrocytes: inhibition of mab with glucoconjugates of lipid and protein nature with different isoelectric properties. Klin Lab Diagn. 2007; (12):48–53. PMID: 18225514.9. Marsh WL. 1972; Scoring of hemagglutination reactions. Transfusion. 12:352–3. DOI: 10.1111/j.1537-2995.1972.tb04459.x. PMID: 5072588.10. Sinha N, Mohan S, Lipschultz CA, Smith-Gill SJ. 2002; Differences in electrostatic properties at antibody-antigen binding sites: implications for specificity and cross-reactivity. Biophys J. 83:2946–68. DOI: 10.1016/S0006-3495(02)75302-2. PMID: 12496069. PMCID: PMC1302377.11. Sinha N, Li Y, Lipschultz CA, Smith-Gill SJ. 2007; Understanding antibody-antigen associations by molecular dynamics simulations: detection of important intra-and inter-molecular salt bridges. Cell Biochem Biophys. 47:361–75. DOI: 10.1007/s12013-007-0031-8. PMID: 17652781.12. Kapingidza AB, Kowal K, Chruszcz M. 2020; Antigen-antibody complexes. Subcell Biochem. 94:465–97. DOI: 10.1007/978-3-030-41769-7_19. PMID: 32189312.13. Yoshida K, Kuroda D, Kiyoshi M, Nakakido M, Nagatoishi S, Soga S, et al. 2019; Exploring designability of electrostatic complementarity at an antigen-antibody interface directed by mutagenesis, biophysical analysis, and molecular dynamics simulations. Sci Rep. 9:4482. DOI: 10.1038/s41598-019-40461-5. PMID: 30872635. PMCID: PMC6418251.14. Gupta P, Makowski EK, Kumar S, Zhang Y, Scheer JM, Tessier PM. 2022; Antibodies with weakly basic isoelectric points minimize trade-offs between formulation and physiological colloidal properties. Mol Pharm. 19:775–87. DOI: 10.1021/acs.molpharmaceut.1c00373. PMID: 35108018. PMCID: PMC9350878.15. Tang Y, Cain P, Anguiano V, Shih JJ, Chai Q, Feng Y. 2021; Impact of IgG subclass on molecular properties of monoclonal antibodies. MAbs. 13:1993768. DOI: 10.1080/19420862.2021.1993768. PMID: 34763607. PMCID: PMC8726687.16. Raut AS, Kalonia DS. 2015; Opalescence in monoclonal antibody solutions and its correlation with intermolecular interactions in dilute and concentrated solutions. J Pharm Sci. 104:1263–74. DOI: 10.1002/jps.24326. PMID: 25556561.17. Neergaard MS, Kalonia DS, Parshad H, Nielsen AD, Møller EH, van de Weert M. 2013; Viscosity of high concentration protein formulations of monoclonal antibodies of the IgG1 and IgG4 subclass - prediction of viscosity through protein-protein interaction measurements. Eur J Pharm Sci. 49:400–10. DOI: 10.1016/j.ejps.2013.04.019. PMID: 23624326.18. Laue T. 2016; Charge matters. Biophys Rev. 8:287–9. DOI: 10.1007/s12551-016-0229-3. PMID: 28510017. PMCID: PMC5425809.19. Liu S, Shah DK. 2023; Physiologically based pharmacokinetic modeling to characterize the effect of molecular charge on whole-body disposition of monoclonal antibodies. AAPS J. 25:48. DOI: 10.1208/s12248-023-00812-7. PMID: 37118220.20. Emoto K, Yamashita S, Okada Y. 2005; Mechanisms of heat-induced antigen retrieval: does pH or ionic strength of the solution play a role for refolding antigens? J Histochem Cytochem. 53:1311–21. DOI: 10.1369/jhc.5C6627.2005. PMID: 16009962.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurement of cyclosporine concentration in whole blood of renal transplant patients: comparison of cyclosporine concentrations determined by radioimmunoassay using specific and nonspecific monoclonal antibodies
- Generation and Characterization of Anti - Human CTLA - 4 Monoclonal Antibodies
- Receptors for murine monoclonal antibodies on the normal blood cells
- Monoclonal antibodies specific to rickettsia typhi
- Monoclonal Antibodies Specific for Treponema Pallidum , Nichols Strain