J Liver Cancer.
2023 Sep;23(2):377-388. 10.17998/jlc.2023.08.24.
Clinical outcome of surgical resection for multifocal T2-T3 hepatocellular carcinoma up to 3 nodules: a comparative analysis with a single nodule
- Affiliations
-
- 1Division of HBP Surgery and Liver Transplantation, Department of Surgery, Korea University College of Medicine, Seoul, Korea
- KMID: 2546417
- DOI: http://doi.org/10.17998/jlc.2023.08.24
Abstract
- Background/Aims
Although the Barcelona Clinic Liver Cancer staging system seems to underestimate the impact of curative-intent surgical resection for multifocal hepatocellular carcinoma (HCC), recent studies have indicated favorable results for the surgical resection of multiple HCC. This study aimed to assess clinical outcomes and feasibility of surgical resection for multifocal HCC with up to three nodules compared with single tumor cases.
Methods
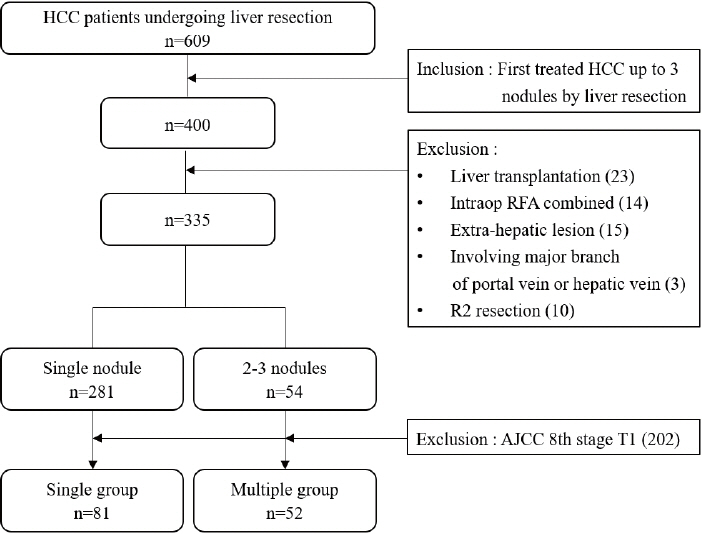
Patients who underwent surgical resection for HCC with up to three nodules between 2009 and 2020 were included, and those with the American Joint Committee on Cancer (AJCC) 8th edition, T1 and T4 stages were excluded to reduce differences in disease distribution and severity. Finally, 81 and 52 patients were included in the single and multiple treatment groups, respectively. Short- and long-term outcomes including recurrence-free survival (RFS) and overall survival (OS), were evaluated.
Results
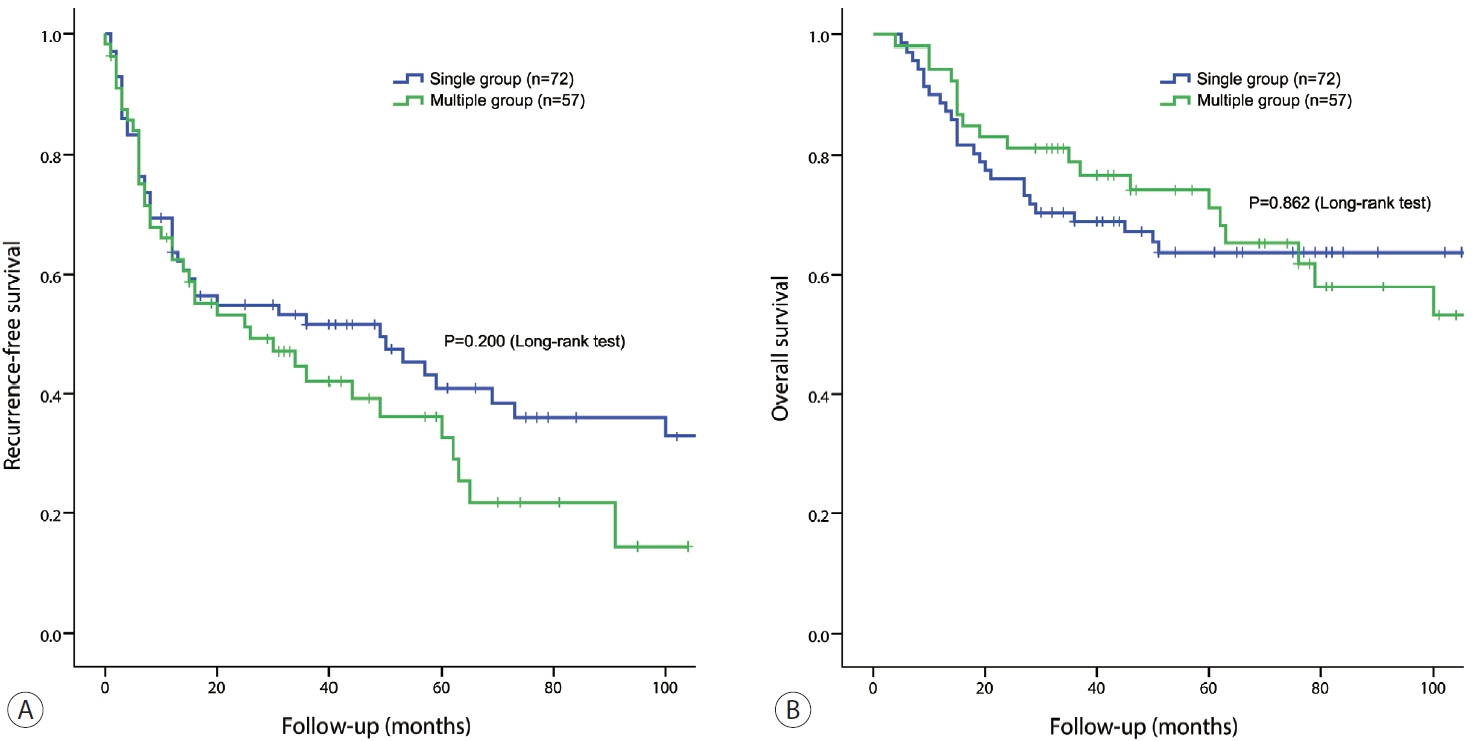
All patients were classified as Child-Pugh class A. RFS and OS were not significantly different between the two groups (P=0.176 and P=0.966, respectively). Multivariate analysis revealed that transfusion and intrahepatic metastasis were significantly associated with recurrence (P=0.046 and P=0.005, respectively). Additionally, intrahepatic metastasis was significantly associated with OS (hazard ratio, 1.989; 95% confidence interval, 1.040-3.802; P=0.038).
Conclusions
Since there was no significant difference in survival between the single and multiple groups among patients with AJCC 8th stage T2 and T3, surgical resection with curative intent could be considered with acceptable long-term survival for selected patients with multiple HCC of up to three nodules.
Figure
Cited by 1 articles
-
Heavy smoking increases early mortality risk in patients with hepatocellular carcinoma after curative treatment
Jaejun Lee, Jong Young Choi, Soon Kyu Lee
J Liver Cancer. 2024;24(2):253-262. doi: 10.17998/jlc.2024.06.02.
Reference
-
References
1. Park HM, Won YJ, Kang MJ, Park SJ, Kim SW, Jung KW, et al. Trend analysis and prediction of hepatobiliary pancreatic cancer incidence and mortality in Korea. J Korean Med Sci. 2022; 37:e216.2. Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008; 134:1908–1916.3. Ho MC, Huang GT, Tsang YM, Lee PH, Chen DS, Sheu JC, et al. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol. 2009; 16:848–855.4. Fukami Y, Kaneoka Y, Maeda A, Kumada T, Tanaka J, Akita T, et al. Liver resection for multiple hepatocellular carcinomas: a Japanese nationwide survey. Ann Surg. 2020; 272:145–154.5. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology. 2016; 150:835–853.6. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999; 19:329–338.7. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022; 76:681–693.8. Varma V, Mehta N, Kumaran V, Nundy S. Indications and contraindications for liver transplantation. Int J Hepatol. 2011; 2011:121862.9. Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection--propensity score analyses of long-term outcomes. Radiology. 2015; 275:908–919.10. Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014; 260:329–340.11. Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, et al. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: a meta-analysis of highquality studies. Hepatology. 2018; 68:977–993.12. Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤ 2 cm in a propensity score model. Ann Surg. 2016; 263:538–545.13. Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol. 2015; 62:617–624.14. Lu L, Zheng P, Wu Z, Chen X. Hepatic resection versus transarterial chemoembolization for intermediate-stage hepatocellular carcinoma: a cohort study. Front Oncol. 2021; 11:618937.15. Zhang DZ, Wei XD, Wang XP. Comparison of hepatic resection and transarterial chemoembolization for solitary hepatocellular carcinoma. World J Gastroenterol. 2015; 21:4635–4643.16. Cassese G, Han HS, Cho JY, Lee HW, Lee B, Troisi RI. Selecting the best approach for the treatment of multiple non-metastatic hepatocellular carcinoma. Cancers (Basel). 2022; 14:5997.17. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer. 2023; 23:1–120.18. Yue YY, Zhou WL. Hepatic resection is associated with improved long-term survival compared to radio-frequency ablation in patients with multifocal hepatocellular carcinoma. Front Oncol. 2020; 10:110.19. Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013; 59:89–97.20. N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009; 50:1475–1483.21. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012; 107:569–577. quiz 578.22. Cucchetti A, Djulbegovic B, Tsalatsanis A, Vitale A, Hozo I, Piscaglia F, et al. When to perform hepatic resection for intermediatestage hepatocellular carcinoma. Hepatology. 2015; 61:905–914.23. Forner A, Gilabert M, Bruix J, Raoul JL. Reply: heterogeneity of intermediate-stage HCC necessitates personalized management including surgery. Nat Rev Clin Oncol. 2015; 12:10.24. Zhaohui Z, Shunli S, Bin C, Shaoqiang L, Yunpeng H, Ming K, et al. Hepatic resection provides survival benefit for selected intermediate-stage (BCLC-B) hepatocellular carcinoma patients. Cancer Res Treat. 2019; 51:65–72.25. Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994; 115:303–309.26. Harada N, Shirabe K, Maeda T, Kayashima H, Ishida T, Maehara Y. Blood transfusion is associated with recurrence of hepatocellular carcinoma after hepatectomy in Child-Pugh class A patients. World J Surg. 2015; 39:1044–1051.27. Kwon AH, Matsui Y, Kamiyama Y. Perioperative blood transfusion in hepatocellular carcinomas: influence of immunologic profile and recurrence free survival. Cancer. 2001; 91:771–778.28. Ydy LR, Slhessarenko N, de Aguilar-Nascimento JE. Effect of perioperative allogeneic red blood cell transfusion on the immuneinflammatory response after colorectal cancer resection. World J Surg. 2007; 31:2044–2051.29. Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012; 256:235–244.30. Wu HL, Tai YH, Lin SP, Chan MY, Chen HH, Chang KY. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4,030 patients. Sci Rep. 2018; 8:13345.31. Li X, Gu B, Wang B, Feng Z, Ma Y, Li H, et al. Intrahepatic metastases may be specific to hepatocellular carcinoma due to the coagulation and fibrinolytic systems (review). Oncol Rep. 2020; 44:2345–2352.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgical Perspectives of Hepatocellular Carcinoma beyond the Barcelona Clinical Liver Cancer Guideline; Focusing on Liver Resection
- Imaging Diagnosis of Hepatocellular Carcinoma
- Hepatocarcinogenesis in liver cirrhosis: imaging diagnosis
- Morphometric Analysis of Cirrhotic Nodules in Hepatocellular Carcinoma-bearing Livers
- The Effect of Presence of Satellite Nodules in Nephrectomized Kidney on Survival in Patients with Renal Cell Carcinoma