J Cerebrovasc Endovasc Neurosurg.
2023 Sep;25(3):316-321. 10.7461/jcen.2023.E2022.03.004.
Awake craniotomy removal of a corticospinal tract developmental venous anomaly hemorrhage: A case report
- Affiliations
-
- 1Department of Neurosurgery, Hospital Privado de Rosario - Grupo Gamma, Rosario, Santa Fe, Argentina
- 2Department of Radiology, Hospital Privado de Rosario - Grupo Gamma, Rosario, Santa Fe, Argentina
- 3Department of Neurophysiology, Hospital Privado de Rosario - Grupo Gamma, Rosario, Santa Fe, Argentina
- KMID: 2546169
- DOI: http://doi.org/10.7461/jcen.2023.E2022.03.004
Abstract
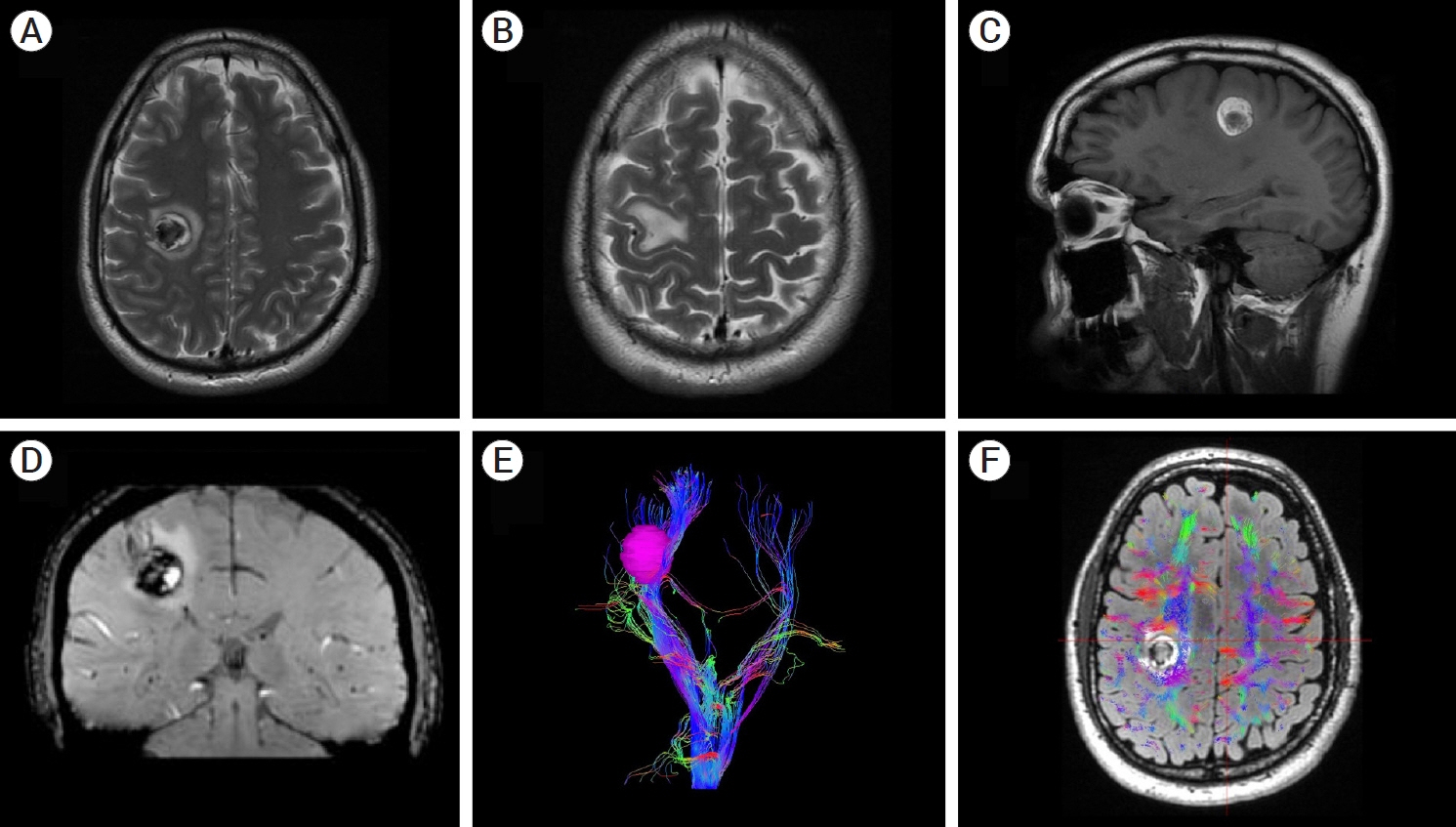
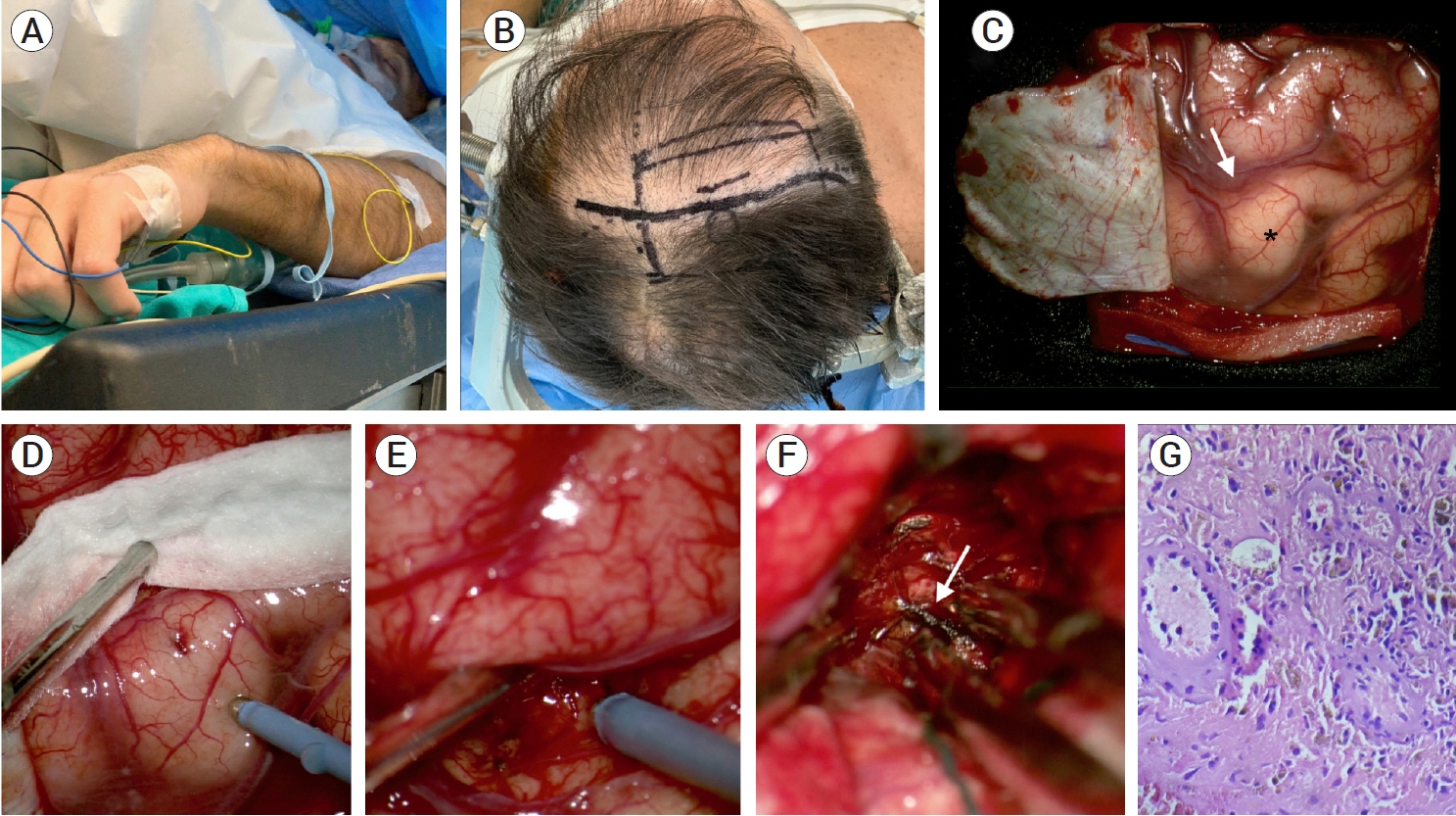
- Developmental venous anomalies (DVAs) are composed of mature venous vessels that lack malformed or neoplastic elements. Although the hemorrhage risk is considered negligible, some patients may have neurological symptoms attributable to acute infarction or intracranial hemorrhage secondary to thrombosis, in the absence of a coexisting cavernous malformation. We report the case of a 42-year-old patient who presented with acute left-hand paresis secondary to a subcortical hemorrhage. This bleeding originated from a DVA in the corticospinal tract area and was surgically drained through an awake craniotomy. To accomplish this, we used a trans-precentral sulcus approach. After the complete removal of the coagulum, small venous channels appeared, which were coagulated. No associated cavernoma was found. Although the main DVA trunk was left patent, no signs of ischemia or venous infarction were observed after coagulating the small venous channels found inside the hematoma cavity. Two weeks after the procedure, the patient’s hand function improved, and he was able to resume desktop work. DVA-associated hemorrhage within the cortico-spinal tract could be safely removed with modern awake mapping techniques. This technique allowed the patient to rapidly improve his hand function.
Keyword
Figure
Reference
-
1. Barrenechea IJ, Rojas H, Nicola M, Marquez L, Herrera R, Van Isseldyk F. A novel temporary cranial fixation device for awake cranial surgery: technical report of 14 cases. Surg Neurol Int. 2020; Jan. 11:12.2. Frigeri T, Paglioli E, de Oliveira E, Rhoton AL Jr. Microsurgical anatomy of the central lobe. J Neurosurg. 2015; Mar. 122(3):483–98.3. Garner TB, Del Curling O Jr, Kelly DL Jr, Laster DW. The natural history of intracranial venous angiomas. J Neurosurg. 1991; Nov. 75(5):715–22.4. Gempt J, Krieg SM, Hüttinger S, Buchmann N, Ryang YM, Shiban E, et al. Postoperative ischemic changes after glioma resection identified by diffusion-weighted magnetic resonance imaging and their association with intraoperative motor evoked potentials. J Neurosurg. 2013; Oct. 119(4):829–36.5. Hill BD, Barkemeyer CA, Jones GN, Santa Maria MP, Minor KS, Browndyke JN. Validation of the coin rotation test: a simple, inexpensive, and convenient screening tool for impaired psychomotor processing speed. Neurologist. 2010; 16(4):249–53.6. Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so-called venous angioma. Neurosurg Rev. 1986; Sep. 9(3):233–42.7. McLaughlin MR, Kondziolka D, Flickinger JC, Lunsford S, Lunsford LD. The prospective natural history of cerebral venous malformations. Neurosurgery. 1998; Aug. 43(2):195–200. discussion 200-1.8. Naff NJ, Wemmer J, Hoenig-Rigamonti K, Rigamonti DR. A longitudinal study of patients with venous malformations: documentation of a negligible hemorrhage risk and benign natural history. Neurology. 1998; Jun. 50(6):1709–14.9. Rabinov JD. Diagnostic imaging of angiographically occult vascular malformations. Neurosurg Clin N Am. 1999; Jul. 10(3):419–32.10. Saito Y, Kobayashi N. Cerebral venous angiomas: clinical evaluation and possible etiology. Radiology. 1981; Apr. 139(1):87–94.11. Sanmillan JL, Fernández-Coello A, Fernández-Conejero I, Plans G, Gabarrós A. Functional approach using intraoperative brain mapping and neurophysiological monitoring for the surgical treatment of brain metastases in the central region. J Neurosurg. 2017; Mar. 126(3):698–707.12. Sarwar M, McCormick WF. Intracerebral venous angioma. Arch Neurol. 1978; May. 35(5):323–5.13. Walter J, Kuhn SA, Waschke A, Kalff R, Ewald C. Operative treatment of subcortical metastatic tumours in the central region. J Neurooncol. 2011; Jul. 103(3):567–73.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Developmental Venous Anomaly Presenting Intracranial Hemorrhage without Associated Vascular Anomaly
- Repeated Intracerebral Hemorrhage from Developmental Venous Anomaly Alone
- Atypical Developmental Venous Anomaly Associated with Single Arteriovenous Fistula and Intracerebral Hemorrhage: a Case Demonstrated by Superselective Angiography
- Joubert Syndrome Presenting With Normal Pyramidal Decussation: A Case Report
- Cerebellar Dysfunctions Associated with a Developmental Venous Anomaly