Ann Surg Treat Res.
2023 Sep;105(3):133-140. 10.4174/astr.2023.105.3.133.
Donor sex and donor-recipient sex disparity do not affect hepatocellular carcinoma recurrence after living donor liver transplantation
- Affiliations
-
- 1Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2545904
- DOI: http://doi.org/10.4174/astr.2023.105.3.133
Abstract
- Purpose
Studies have yielded contradictory results on whether donor sex and donor-recipient sex disparity affect hepatocellular carcinoma (HCC) recurrence after living donor liver transplantation (LDLT). The present study assessed whether donor sex or donor-recipient sex disparity affects HCC recurrence after LDLT at a high-volume center.
Methods
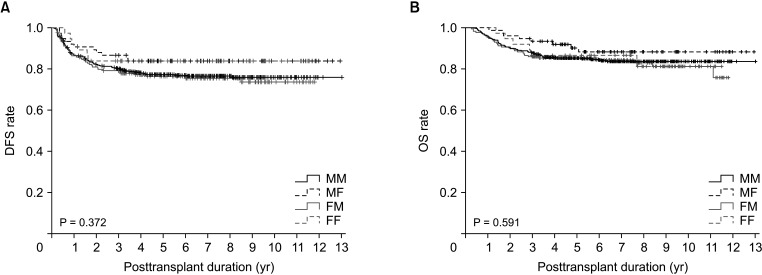
This study included 772 HCC patients who underwent LDLT between January 2006 and December 2015 at Asan Medical Center. Patients were divided into 4 groups based on the sex of the donor and recipient: male-to-male (n = 490, 63.5%), male-to-female (n = 75, 9.7%), female-to-male (n = 170, 22.0%), and female-to-female (n = 37, 4.8%).
Results
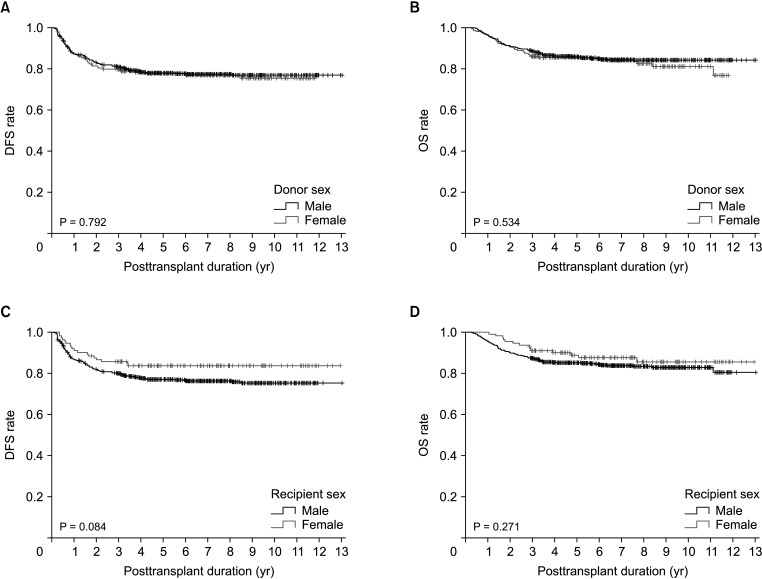
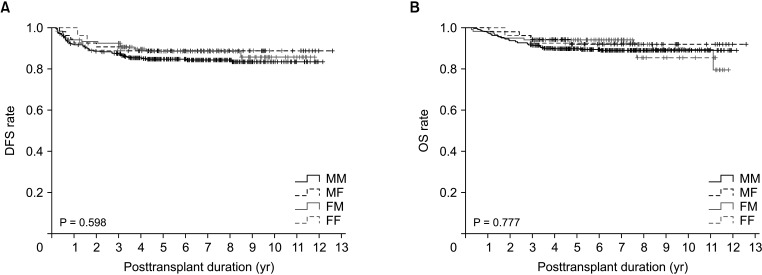
Disease-free survival (DFS; P = 0.372) and overall survival (OS; P = 0.591) did not differ significantly among the 4 groups. DFS also did not differ significantly between LDLT recipients with male and female donors (P = 0.792) or between male and female recipients (P = 0.084). After patient matching with an α-FP/des-γ-carboxy prothrombin/tumor volume score cutoff of 5logs, donor-recipient sex disparity did not significantly affect DFS (P = 0.598) or OS (P = 0.777). There were also no differences in DFS in matched LDLT recipients with male and female donors (P = 0.312) or between male and female recipients (P = 0.374).
Conclusion
Neither donor sex nor donor-recipient sex disparity significantly affected posttransplant HCC recurrence.
Figure
Reference
-
1. Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017; 3:524–548. PMID: 27918777.2. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019; 70:151–171. PMID: 30266282.
Article3. Giannini EG, Farinati F, Ciccarese F, Pecorelli A, Rapaccini GL, Di Marco M, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015; 61:184–190. PMID: 25234419.
Article4. Zhang H, Han J, Xing H, Li ZL, Schwartz ME, Zhou YH, et al. Sex difference in recurrence and survival after liver resection for hepatocellular carcinoma: a multicenter study. Surgery. 2019; 165:516–524. PMID: 30337048.
Article5. Han S, Yang JD, Sinn DH, Kim JM, Choi GS, Jung G, et al. Risk of post-transplant hepatocellular carcinoma recurrence is higher in recipients of livers from male than female living donors. Ann Surg. 2018; 268:1043–1050. PMID: 28628564.
Article6. Nakamura T, Sasaki K, Kojima L, Teo R, Inaba Y, Yamamoto T, et al. Impact of donor sex on hepatocellular carcinoma recurrence in liver transplantation after brain death. Clin Transplant. 2023; e14989. PMID: 37039506.
Article7. Taura K, Shimamura T, Akamatsu N, Umeshita K, Fujiyoshi M, Abe H, et al. No impact of donor sex on the recurrence of hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Sci. 2022; 29:570–584. PMID: 35279950.
Article8. Hwang S, Song GW, Lee YJ, Kim KH, Ahn CS, Moon DB, et al. Multiplication of tumor volume by two tumor markers is a post-resection prognostic predictor for solitary hepatocellular carcinoma. J Gastrointest Surg. 2016; 20:1807–1820. PMID: 27311982.
Article9. Jung DH, Hwang S, Lee YJ, Kim KH, Song GW, Ahn CS, et al. Small hepatocellular carcinoma with low tumor marker expression benefits more from anatomical resection than tumors with aggressive biology. Ann Surg. 2019; 269:511–519. PMID: 28837444.
Article10. Hwang S, Song GW, Ahn CS, Kim KH, Moon DB, Ha TY, et al. Quantitative prognostic prediction using ADV score for hepatocellular carcinoma following living donor liver transplantation. J Gastrointest Surg. 2021; 25:2503–2515. PMID: 33532981.
Article11. Park GC, Hwang S, You YK, Choi Y, Kim JM, Joo DJ, et al. Quantitative prediction of posttransplant hepatocellular carcinoma prognosis using ADV score: validation with Korea-nationwide transplantation registry database. J Gastrointest Surg. 2023; 27:1353–1366. PMID: 37039979.
Article12. Hemming AW, Cattral MS, Reed AI, Van Der Werf WJ, Greig PD, Howard RJ. Liver transplantation for hepatocellular carcinoma. Ann Surg. 2001; 233:652–659. PMID: 11323504.
Article13. Leung JY, Zhu AX, Gordon FD, Pratt DS, Mithoefer A, Garrigan K, et al. Liver transplantation outcomes for early-stage hepatocellular carcinoma: results of a multicenter study. Liver Transpl. 2004; 10:1343–1354. PMID: 15497158.
Article14. Regalia E, Fassati LR, Valente U, Pulvirenti A, Damilano I, Dardano G, et al. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat Surg. 1998; 5:29–34. PMID: 9683751.
Article15. Schlitt HJ, Neipp M, Weimann A, Oldhafer KJ, Schmoll E, Boeker K, et al. Recurrence patterns of hepatocellular and fibrolamellar carcinoma after liver transplantation. J Clin Oncol. 1999; 17:324–331. PMID: 10458250.
Article16. Zavaglia C, De Carlis L, Alberti AB, Minola E, Belli LS, Slim AO, et al. Predictors of long-term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol. 2005; 100:2708–2716. PMID: 16393224.
Article17. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–699. PMID: 8594428.
Article18. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001; 33:1394–1403. PMID: 11391528.
Article19. Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008; 14:935–945. PMID: 18581465.
Article20. Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009; 49:832–838. PMID: 19152426.
Article21. Fujiki M, Takada Y, Ogura Y, Oike F, Kaido T, Teramukai S, et al. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant. 2009; 9:2362–2371. PMID: 19656125.
Article22. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009; 10:35–43. PMID: 19058754.
Article23. Ma WL, Hsu CL, Wu MH, Wu CT, Wu CC, Lai JJ, et al. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008; 135:947–955.e9555. PMID: 18639551.
Article24. Acosta-Lopez S, Diaz-Bethencourt D, Concepción-Massip T, Martin-Fernandez de Basoa MC, Plata-Bello A, Gonzalez-Rodriguez A, et al. The androgen receptor expression and its activity have different relationships with prognosis in hepatocellular carcinoma. Sci Rep. 2020; 10:22046. PMID: 33328560.
Article25. Ao J, Meng J, Zhu L, Nie H, Yang C, Li J, et al. Activation of androgen receptor induces ID1 and promotes hepatocellular carcinoma cell migration and invasion. Mol Oncol. 2012; 6:507–515. PMID: 22819717.
Article26. Boix L, Castells A, Bruix J, Solé M, Brú C, Fuster J, et al. Androgen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resection. J Hepatol. 1995; 22:616–622. PMID: 7560855.
Article27. Wu MH, Ma WL, Hsu CL, Chen YL, Ou JH, Ryan CK, et al. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci Transl Med. 2010; 2:32ra35.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Donor sex and donor-recipient sex disparity do not affect hepatocellular carcinoma recurrence after living donor liver transplantation
- Liver retransplantation for adult recipients
- Minimal-incision donor right hepatectomy for living donor liver transplantation
- Complete transition from open to laparoscopic living donor hepatectomy: 8-year experience with more than 500 laparoscopy cases
- Liver stiffness measurement and outcomes of living donor liver transplantation