Obstet Gynecol Sci.
2023 Sep;66(5):407-416. 10.5468/ogs.23119.
Pretreatment total lymphocyte count as a prognostic factor of survival in patients with recurrent cervical cancer after definitive radiation-based therapy: a retrospective study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Songklanagarind Hospital, Songkhla, Thailand
- 2Department of Biomedical Sciences and Biomedical Engineering, Songklanagarind Hospital, Songkhla, Thailand
- 3Translational Medicine Research Center, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand
- KMID: 2545887
- DOI: http://doi.org/10.5468/ogs.23119
Abstract
Objective
This study evaluated the association between pretreatment total lymphocyte count (TLC) and overall survival (OS) in patients with recurrent cervical cancer.
Methods
We retrospectively reviewed 290 patients with recurrent cervical cancer with definite complete responses to either definitive radiotherapy or concurrent chemoradiotherapy between January 2009 and December 2022. The associations between pretreatment TLC and progression-free survival (PFS) and OS rates were evaluated.
Results
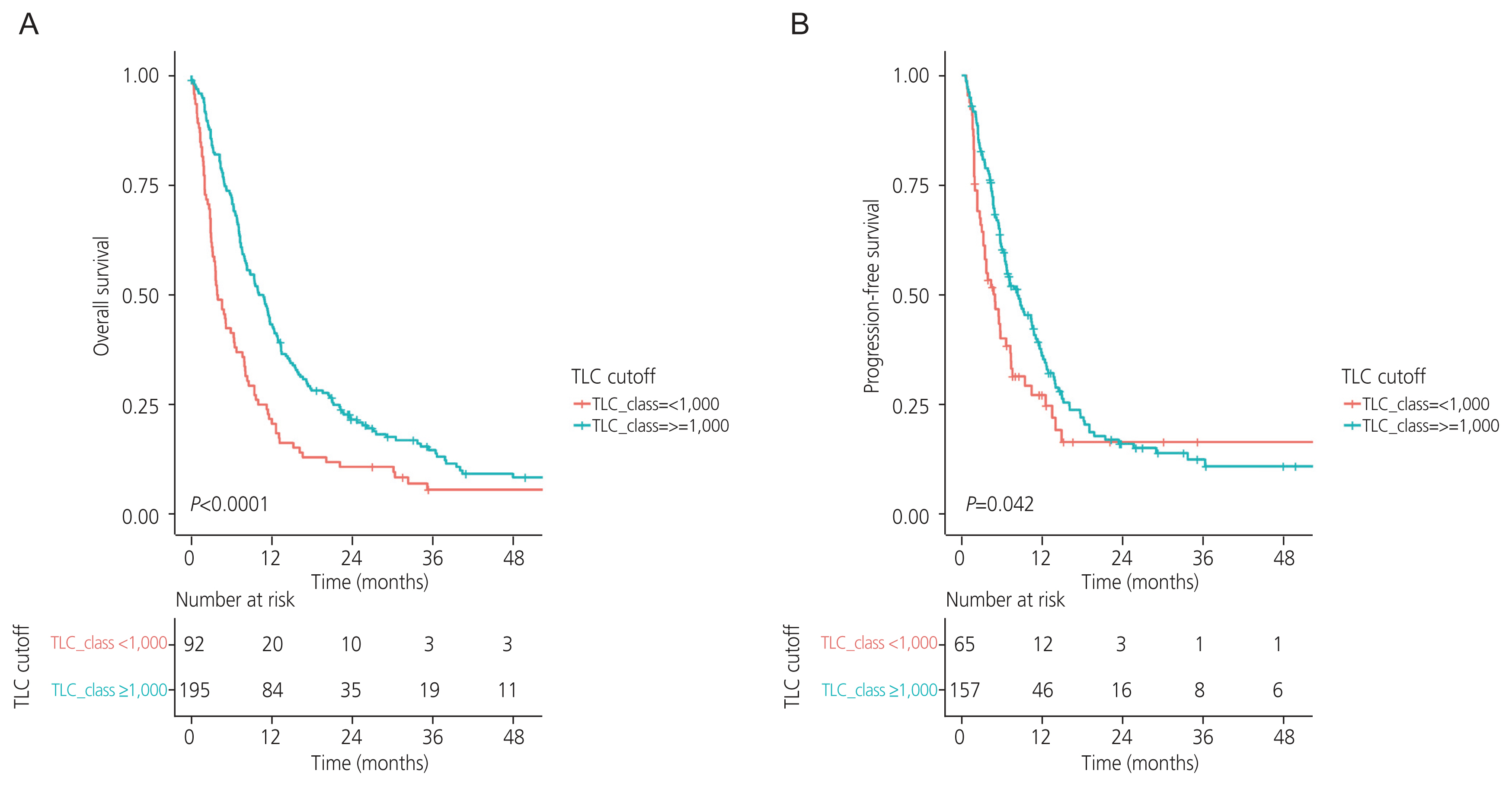
Ninety-three patients (32%) had a pretreatment TLC <1,000 cells/mm3. Patients with a pretreatment TLC <1,000 cells/mm3 had lower treatment response rates than their counterparts (P=0.045). The OS and PFS rates were significantly higher in patients with pretreatment TLC ≥1,000 cells/mm3 than in those with pretreatment TLC <1,000 cells/mm3 (10.74 vs. 3.89 months, P<0.0001; 8.32 vs. 4.97 months, P=0.042; respectively). Moreover, pretreatment TLC ≥1,000 cells/mm3 was identified as an independent prognostic factor for OS in both univariate analysis (hazard ratio [HR], 0.57; 95% conficence interval [CI], 0.44-0.74; P<0.001) and multivariate analysis (HR, 0.64; 95% CI, 0.47-0.86; P=0.003). However, TLC ≥1,000 cells/mm3 was identified as a prognostic factor for PFS only in univariate analysis (HR, 0.71; 95% CI, 0.51-0.99; P=0.043) but not in the multivariate analysis (HR, 0.81; 95% CI, 0.55-1.18; P=0.3).
Conclusion
Pretreatment TLC was associated with treatment response and was identified as an independent prognostic factor associated with the survival outcomes of patients with recurrent cervical cancer.
Keyword
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.2. ThaiCancerBase National Cancer Institute. Hospital-based cancer registry 2020 [Internet]. Bangkok (TH): ThaiCancerBase National Cancer Institute;c2020. [cited 2022 Dec 26]. Available from: https://www.nci.go.th/e_book/hosbased_2563/index.html .3. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021; 155(Suppl 1):28–44.4. Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992; 24:197–204.5. Eifel PJ, Jhingran A, Brown J, Levenback C, Thames H. Time course and outcome of central recurrence after radiation therapy for carcinoma of the cervix. Int J Gynecol Cancer. 2006; 16:1106–11.6. Poolkerd S, Leelahakorn S, Manusirivithaya S, Tangjitgamol S, Thavaramara T, Sukwattana P, et al. Survival rate of recurrent cervical cancer patients. J Med Assoc Thai. 2006; 89:275–82.7. Thanagumtorn K. Survival rate of recurrent cervical carcinoma. J Med Assoc Thai. 2012; 95(Suppl 3):S125–30.8. Taguchi A, Nakajima Y, Furusawa A, Yoshino Y, Takao M, Kashiyama T, et al. High neutrophil-to-lymphocyte ratio is a predictor of short-term survival for patients with recurrent cervical cancer after radiation-based therapy. J Obstet Gynaecol Res. 2021; 47:1862–70.9. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: cervical cancer version 1, 2023 [Internet]. Plymouth Meeting (PA): National Comprehensive Cancer Network;c2023. [cited 2023 Mar 15]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1426 .10. Rochet NM, Markovic SN, Porrata LF. The role of complete blood cell count in prognosis-watch this space! Oncol Hematol Rev. 2012; 8:76–82.11. Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017; 14:155–67.12. Shimizu K, Iyoda T, Okada M, Yamasaki S, Fujii SI. Immune suppression and reversal of the suppressive tumor microenvironment. Int Immunol. 2018; 30:445–54.13. Wu ES, Oduyebo T, Cobb LP, Cholakian D, Kong X, Fader AN, et al. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol. 2016; 140:76–82.14. Shi F, Yoder AK, Mach C, Dalwadi S, Anderson ML, Hall TR, et al. Impact of hematologic toxicities during concurrent chemoradiation for cervical cancer. Obstet Gynecol Sci. 2022; 65:176–87.15. Thiangphak E, Pruegsanusak K, Buhachat R. Pretreatment lymphocyte count as independent prognostic factor in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy. Int J Gynaecol Obstet. 2022; 159:672–8.16. Inoue H, Shiozaki A, Fujiwara H, Konishi H, Kiuchi J, Ohashi T, et al. Absolute lymphocyte count and C-reactive protein-albumin ratio can predict prognosis and adverse events in patients with recurrent esophageal cancer treated with nivolumab therapy. Oncol Lett. 2022; 24:257.17. Zhang Y, Dai K, Zhang Q, Huang Y, Feng Y, Bhardwaj D, et al. Normal absolute monocyte count in combination with normal/high absolute lymphocyte count at the time of relapse is associated with improved survival in patients with early relapsed acute myeloid leukemia. Cancer Invest. 2021; 39:550–8.18. Park JC, Durbeck J, Clark JR. Predictive value of peripheral lymphocyte counts for immune checkpoint inhibitor efficacy in advanced head and neck squamous cell carcinoma. Mol Clin Oncol. 2020; 13:87.19. Ida N, Nakamura K, Saijo M, Kusumoto T, Masuyama H. Prognostic nutritional index as a predictor of survival in patients with recurrent cervical cancer. Mol Clin Oncol. 2018; 8:257–63.20. Ittiamornlert P, Ruengkhachorn I. Neutrophil-lymphocyte ratio as a predictor of oncologic outcomes in stage IVB, persistent, or recurrent cervical cancer patients treated by chemotherapy. BMC Cancer. 2019; 19:51.21. Vasu S, Caligiuri MA. Lymphocytosis and lymphocytopenia. Kaushansky K, Lichtman MA, Prchal JT, Levi M, Press OW, Burns LJ, editors. Williams hematology. 9th ed. New York (NY): McGraw-Hill Education;2015. p. 1204.22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.23. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–64.24. Maleki Vareki S, Garrigós C, Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol. 2017; 116:116–24.25. Ho WJ, Yarchoan M, Hopkins A, Mehra R, Grossman S, Kang H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J Immunother Cancer. 2018; 6:84.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Prognostic Value of the Preoperative Lymphocyte Count in Patients with Gastric Cancer
- Prognostic significance of pretreatment lymphocyte percentage and age at diagnosis in patients with locally advanced cervical cancer treated with definite radiotherapy
- Blood Lymphocytes as a Prognostic Factor for Stage III Non-Small Cell Lung Cancer with Concurrent Chemoradiation
- Effect of Radiation Therapy for Urogenital Malignant Tumors on Peripheral Lymphocyte Count
- The Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio Are Prognostic Factors in Patients with Locally Advanced Pancreatic Cancer Treated with Chemoradiotherapy