J Korean Med Sci.
2023 Sep;38(36):e280. 10.3346/jkms.2023.38.e280.
Risk Factors for the Prescription of Ineffective Antiviral Candidates for COVID-19 During the Early Pandemic Period in Korea
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 2Division of Infectious Diseases, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 3Transdisciplinary Department of Medicine and Advanced Technology, Seoul National University Hospital, Seoul, Korea
- 4College of Pharmacy, Dankook University, Cheonan, Korea
- 5Public Healthcare Center, Seoul National University Hospital, Seoul, Korea
- 6Department of Emergency Medicine, Seoul National University College of Medicine, Seoul, Korea
- 7Department of Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2545782
- DOI: http://doi.org/10.3346/jkms.2023.38.e280
Abstract
- Background
Although the evidence of treatment for coronavirus disease 2019 (COVID-19) changed rapidly, little is known about the patterns of potential pharmacological treatment during the early period of the COVID-19 pandemic in Korea and the risk factors for ineffective prescription.
Methods
Using claims data from the Korean National Health Insurance System, this retrospective cohort study included admission episodes for COVID-19 from February to December 2020. Ineffective antiviral prescriptions for COVID-19 were defined as lopinavir/ ritonavir (LPN/r) and hydroxychloroquine (HCQ) prescribed after July 2020, according to the revised National Institute of Health COVID-19 treatment guidelines. Factors associated with ineffective prescriptions, including patient and hospital factors, were identified by multivariate logistic regression analysis.
Results
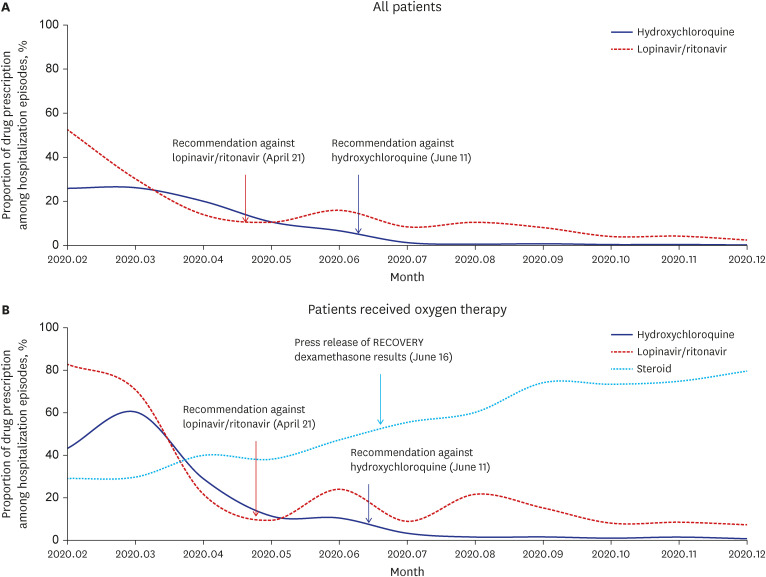
Of the 15,723 COVID-19 admission episodes from February to June 2020, 4,183 (26.6%) included prescriptions of LPN/r, and 3,312 (21.1%) included prescriptions of HCQ. Of the 48,843 admission episodes from July to December 2020, after the guidelines were revised, 2,258 (4.6%) and 182 (0.4%) included prescriptions of ineffective LPN/r and HCQ, respectively. Patient factors independently associated with ineffective antiviral prescription were older age (adjusted odds ratio [aOR] per 10-year increase, 1.17; 95% confidence interval [CI], 1.14–1.20) and severe condition with an oxygen requirement (aOR, 2.49; 95% CI, 2.24–2.77). The prescription of ineffective antiviral drugs was highly prevalent in primary and nursing hospitals (aOR, 40.58; 95% CI, 31.97–51.50), public sector hospitals (aOR, 15.61; 95% CI, 12.76–19.09), and regions in which these drugs were highly prescribed before July 2020 (aOR, 10.65; 95% CI, 8.26–13.74).
Conclusion
Ineffective antiviral agents were prescribed to a substantial number of patients during the first year of the COVID-19 pandemic in Korea. Treatment with these ineffective drugs tended to be prolonged in severely ill patients and in primary and public hospitals.
Keyword
Figure
Reference
-
1. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020; 35(13):e142. PMID: 32242348.
Article2. Suh HJ, Kim DH, Heo EY, Lee HW, Lee JK, Lee CS, et al. Clinical characteristics of COVID-19: clinical dynamics of mild severe acute respiratory syndrome coronavirus 2 infection detected by early active surveillance. J Korean Med Sci. 2020; 35(32):e297. PMID: 32808513.
Article3. Kim PS, Read SW, Fauci AS. Therapy for early COVID-19: a critical need. JAMA. 2020; 324(21):2149–2150. PMID: 33175121.4. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, January 28, 2020. Accessed April 25, 2023. https://apps.who.int/iris/handle/10665/330893 .5. World Health Organization. Therapeutics and COVID-19: living guideline, November 20, 2020. Accessed April 25, 2023. https://apps.who.int/iris/handle/10665/336729 .6. National Institutes of Health COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. April 21, 2020. Accessed April 25, 2023. https://www.covid19treatmentguidelines.nih.gov/ .7. The National Institute for Health and Care Excellence (NICE) guideline: COVID-19 rapid guideline: managing COVID-19, March 23, 2021. Accessed April 25, 2023. https://www.nice.org.uk/guidance/ng191 .8. Jeon J, Chin B. Treatment options for patients with mild-to-moderate coronavirus disease 2019 in Korea. J Korean Med Sci. 2022; 37(48):e352. PMID: 36513054.
Article9. Park DH, Kang CK, Choe PG, Kim NJ, Park WB, Oh MD. How we have treated severe to critically ill patients with coronavirus disease 2019 in Korea. J Korean Med Sci. 2022; 37(49):e353. PMID: 36536547.
Article10. WHO Solidarity Trial Consortium. Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, et al. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N Engl J Med. 2021; 384(6):497–511. PMID: 33264556.
Article11. FDA News Release. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine, June 15, 2020. Accessed April 25, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and#:~:text=Today%2C%20the%20U.S.%20Food%20and,clinical%20trial%20was%20unavailable%2C%20or .12. Kang CK, Seong MW, Choi SJ, Kim TS, Choe PG, Song SH, et al. In vitro activity of lopinavir/ritonavir and hydroxychloroquine against severe acute respiratory syndrome coronavirus 2 at concentrations achievable by usual doses. Korean J Intern Med. 2020; 35(4):782–787. PMID: 32460458.
Article13. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020; 382(19):1787–1799. PMID: 32187464.14. Riva L, Yuan S, Yin X, Martin-Sancho L, Matsunaga N, Pache L, et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020; 586(7827):113–119. PMID: 32707573.
Article15. Weston S, Coleman CM, Haupt R, Logue J, Matthews K, Li Y, et al. Broad anti-coronavirus activity of food and drug administration-approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. J Virol. 2020; 94(21):e01218-20. PMID: 32817221.
Article16. Berendes S, Heywood P, Oliver S, Garner P. Quality of private and public ambulatory health care in low and middle income countries: systematic review of comparative studies. PLoS Med. 2011; 8(4):e1000433. PMID: 21532746.
Article17. Basu S, Andrews J, Kishore S, Panjabi R, Stuckler D. Comparative performance of private and public healthcare systems in low- and middle-income countries: a systematic review. PLoS Med. 2012; 9(6):e1001244. PMID: 22723748.
Article18. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020; 395(10223):473–475. PMID: 32043983.19. Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020; 395(10225):683–684. PMID: 32122468.
Article20. RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021; 384(8):693–704. PMID: 32678530.
Article21. World Health Organization. Therapeutics and COVID-19: living guideline. September 2, 2020. Accessed April 25, 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2022.4 .22. The National Institutes of Health COVID-19 Treatment Guidelines Panel provides recommendations for dexamethasone in patients with COVID-19. Updated June 25, 2020. Accessed April 25, 2023. https://files.covid19treatmentguidelines.nih.gov/guidelines/archive/recommendations-for-dexametha-06-25-2020.pdf .23. Watanabe JH, Kwon J, Nan B, Abeles SR, Jia S, Mehta SR. Medication use patterns in hospitalized patients with COVID-19 in California during the pandemic. JAMA Netw Open. 2021; 4(5):e2110775. PMID: 34019090.24. Nori P, Bartash R, Cowman K, Dackis M, Pirofski LA. Is burnout infectious? Understanding drivers of burnout and job satisfaction among academic infectious diseases physicians. Open Forum Infect Dis. 2019; 6(4):ofz092. PMID: 31041336.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antivirals for Coexistence with COVID-19: Brief Review for General Physicians
- COVID-19 Antiviral and Treatment Candidates: Current Status
- Small Molecule Drug Candidates for Managing the Clinical Symptoms of COVID-19: a Narrative Review
- The Management of Thyroid Disease in COVID-19 Pandemic
- The COVID-19 pandemic's impact on prostate cancer screening and diagnosis in Korea