J Korean Med Sci.
2023 Sep;38(35):e278. 10.3346/jkms.2023.38.e278.
Developing Operational Definitions Related to Helicobacter pylori Eradication Therapy
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea
- 2College of Pharmacy, Chung-Ang University, Seoul, Korea
- 3Department of Global Innovative Drugs, The Graduate School of Chung-Ang University, Seoul, Korea
- 4Division of Gastroenterology, Department of Internal Medicine and Gastrointestinal Cancer Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Division of Gastroenterology, Department of Internal Medicine, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 6Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 7Division of Gastroenterology, Department of Internal Medicine, Inje University Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea
- 8Department of Internal Medicine, Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea
- 9Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2545556
- DOI: http://doi.org/10.3346/jkms.2023.38.e278
Abstract
- Background
The lack of well-established operational definitions is a major limitation of Helicobacter pylori eradication studies that use secondary databases. We aimed to develop and validate operational definitions related to H. pylori eradication therapy.
Methods
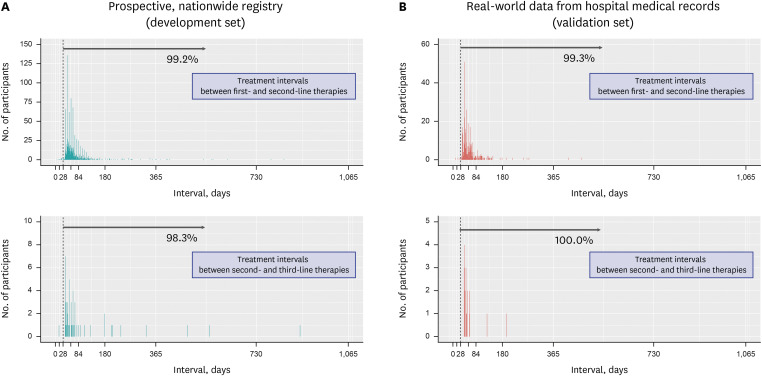
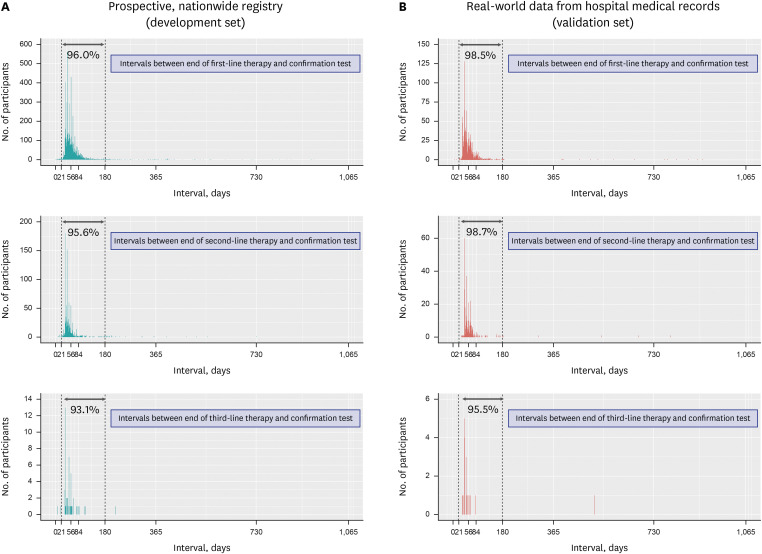
Operational definitions were developed by analyzing a nationwide H. pylori eradication registry and validated using real-world data from hospital medical records. The primary endpoint was the sensitivity of the operational definitions in identifying individuals who received H. pylori eradication therapy. The secondary endpoint was the sensitivity and specificity of the operational definition in identifying successful H. pylori eradication therapy.
Results
H. pylori eradication therapy was defined as a prescription for one of the following combinations: 1) proton pump inhibitor (PPI) + amoxicillin + clarithromycin, 2) PPI + amoxicillin + metronidazole, 3) PPI + metronidazole + tetracycline, 4) PPI + amoxicillin + levofloxacin, 5) PPI + amoxicillin + moxifloxacin, or 6) PPI + amoxicillin + rifabutin. In the validation set, the sensitivity of the operational definition for identifying individuals who received H. pylori eradication therapy was 99.7% and 99.8% for the first- and second-line therapies, respectively. Operational definition to determine success or failure of the H. pylori eradication therapy was developed based on a confirmatory test and the prescription of rescue therapy. The sensitivity and specificity of the operational definition for predicting successful eradication were 97.6% and 91.4%, respectively, in first-line therapy and 98.6% and 54.8%, respectively, in second-line therapy.
Conclusion
We developed and validated operational definitions related to H. pylori eradication therapy. These definitions will help researchers perform various H. pylori eradication-related studies using secondary databases.
Figure
Reference
-
1. Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. 2011; 20(3):299–304. PMID: 21961099.2. McColl KEL. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010; 362(17):1597–1604. PMID: 20427808.3. de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012; 13(6):607–615. PMID: 22575588.
Article4. He J, Liu Y, Ouyang Q, Li R, Li J, Chen W, et al. Helicobacter pylori and unignorable extragastric diseases: mechanism and implications. Front Microbiol. 2022; 13:972777. PMID: 35992650.
Article5. Gravina AG, Priadko K, Ciamarra P, Granata L, Facchiano A, Miranda A, et al. Extra-gastric manifestations of Helicobacter pylori infection. J Clin Med. 2020; 9(12):3887. PMID: 33265933.6. Kim HJ, Kim YJ, Seo SI, Shin WG, Park CH. Impact of the timing of Helicobacter pylori eradication on the risk of development of metachronous lesions after treatment of early gastric cancer: a population-based cohort study. Gastrointest Endosc. 2020; 92(3):613–622.e1. PMID: 32473251.7. Kim YI, Kim YA, Kim HJ, Kim SH, Hwangbo Y, Kim JG, et al. Effect of Helicobacter pylori treatment on the long-term mortality in patients with type 2 diabetes. Korean J Intern Med. 2021; 36(3):584–595. PMID: 33232592.
Article8. Leung WK, Wong IOL, Cheung KS, Yeung KF, Chan EW, Wong AY, et al. Effects of Helicobacter pylori treatment on incidence of gastric cancer in older individuals. Gastroenterology. 2018; 155(1):67–75. PMID: 29550592.9. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab J. 2014; 38(5):395–403. PMID: 25349827.
Article10. Lee YH, Han K, Ko SH, Ko KS, Lee KU. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Data analytic process of a nationwide population-based study using national health information database established by National Health Insurance Service. Diabetes Metab J. 2016; 40(1):79–82. PMID: 26912157.
Article11. Jang S, Ah YM, Jang S, Kim Y, Lee JY, Kim JH. Potentially inappropriate medication use and associated factors in residents of long-term care facilities: a nationwide cohort study. Front Pharmacol. 2023; 13:1092533. PMID: 36703731.
Article12. Kim BJ, Yang CH, Song HJ, Jeon SW, Kim GH, Kim HS, et al. Online registry for nationwide database of Helicobacter pylori eradication in Korea: correlation of antibiotic use density with eradication success. Helicobacter. 2019; 24(5):e12646. PMID: 31368629.
Article13. Jung HK, Kang SJ, Lee YC, Yang HJ, Park SY, Shin CM, et al. Evidence-based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Gut Liver. 2021; 15(2):168–195. PMID: 33468712.
Article14. Hahm KB, Lee KJ, Kim YS, Kim JH, Cho SW, Yim H, et al. Augmented eradication rates of Helicobacter pylori by new combination therapy with lansoprazole, amoxicillin, and rebamipide. Dig Dis Sci. 1998; 43(2):235–240. PMID: 9512112.15. Jung YS, Park CH, Park JH, Nam E, Lee HL. Efficacy of Helicobacter pylori eradication therapies in Korea: a systematic review and network meta-analysis. Helicobacter. 2017; 22(4):e12389.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Approach to Patients after Successful Eradication of Helicobacter pylori
- Trend for Treatment of Helicobacter pylori in Overseas Country
- Prevention of Gastric Cancer: Helicobacter pylori Treatment
- Indications for Helicobacter pylori Eradication Therapy
- Eradication Therapy for Helicobacter pylori with Diagnostic Test for Clarithromycin Resistance