Int J Stem Cells.
2023 Aug;16(3):304-314. 10.15283/ijsc21242.
Hypoxia Differentially Affects Chondrogenic Differentiation of Progenitor Cells from Different Origins
- Affiliations
-
- 1Normandy University, UNICAEN, EA 7451 BioConnecT, Caen, France
- 2Fédération Hospitalo Universitaire SURFACE, Amiens, Caen, France
- 3Service de chirurgie Maxillo-faciale, CHU de Caen, Caen, France
- 4Clinique Saint Martin, Service de Chirurgie Orthopédique, Caen, France
- 5Service ORL et chirurgie cervico-faciale, CHU de Caen, Caen, France
- KMID: 2545230
- DOI: http://doi.org/10.15283/ijsc21242
Abstract
- Background and Objectives
Ear cartilage malformations are commonly encountered problems in reconstructive surgery, since cartilage has low self-regenerating capacity. Malformations that impose psychological and social burden on one’s life are currently treated using ear prosthesis, synthetic implants or autologous flaps from rib cartilage. These approaches are challenging because not only they request high surgical expertise, but also they lack flexibility and induce severe donor-site morbidity. Through the last decade, tissue engineering gained attention where it aims at regenerating human tissues or organs in order to restore normal functions. This technique consists of three main elements, cells, growth factors, and above all, a scaffold that supports cells and guides their behavior. Several studies have investigated different scaffolds prepared from both synthetic or natural materials and their effects on cellular differentiation and behavior.
Methods and Results
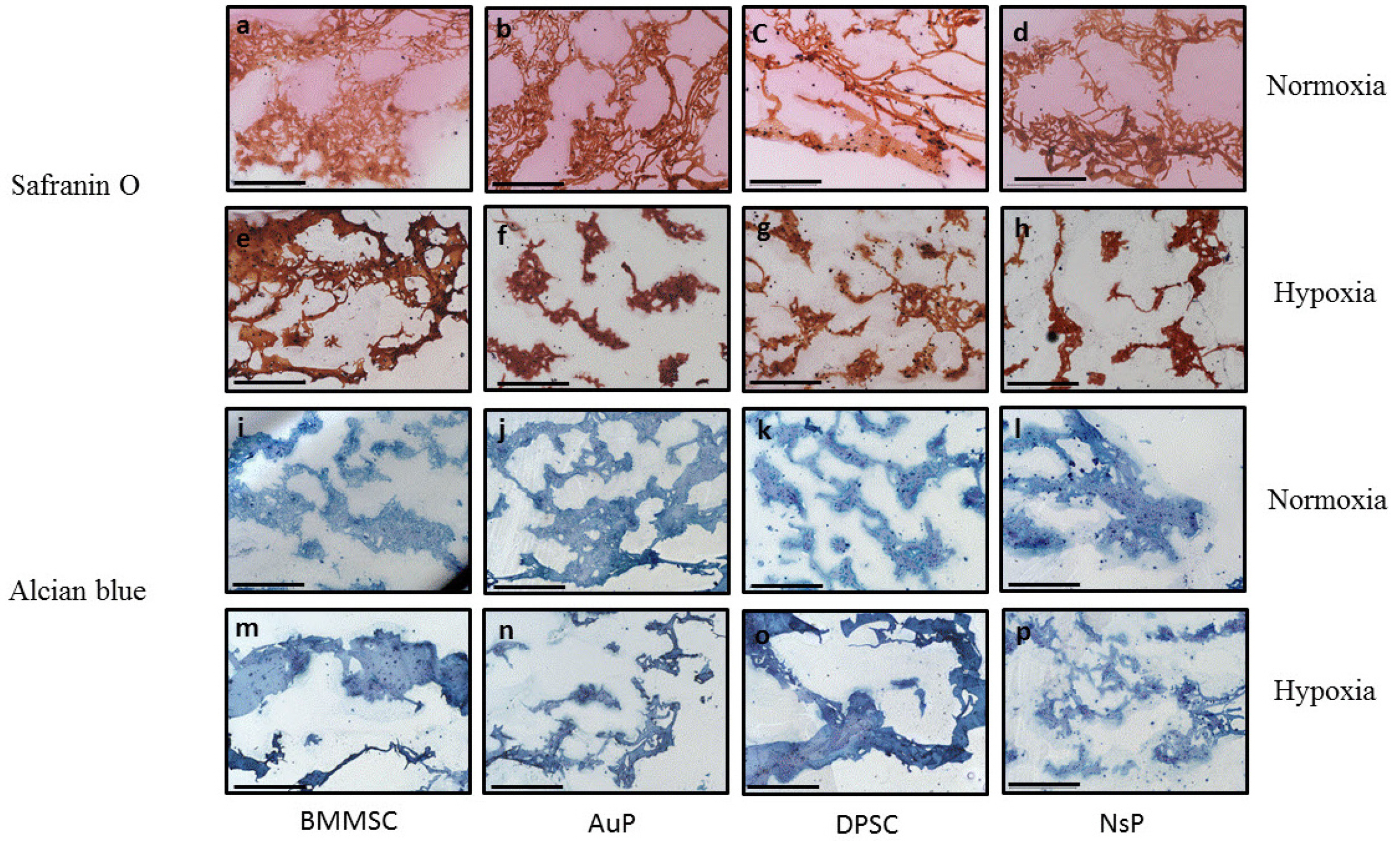
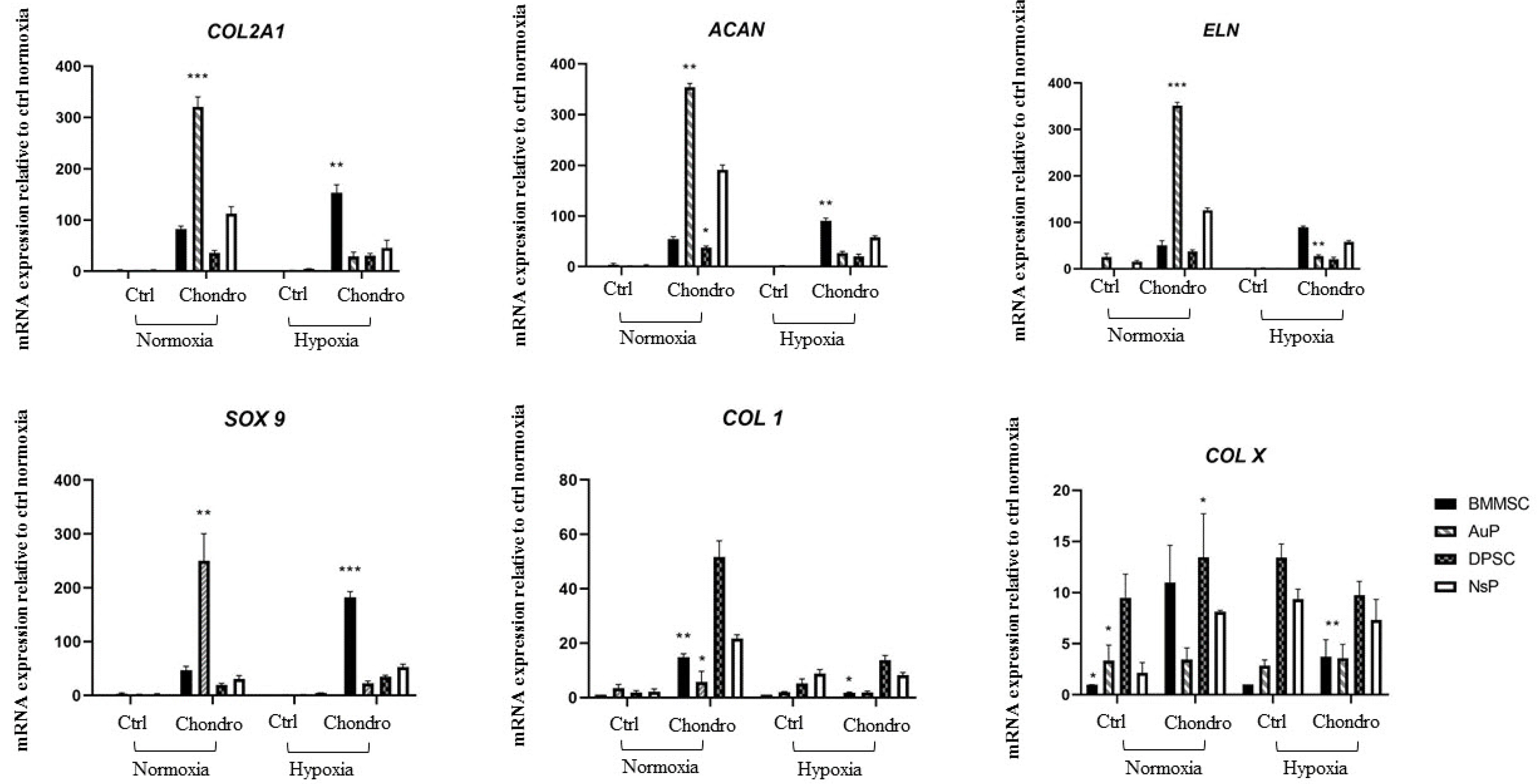
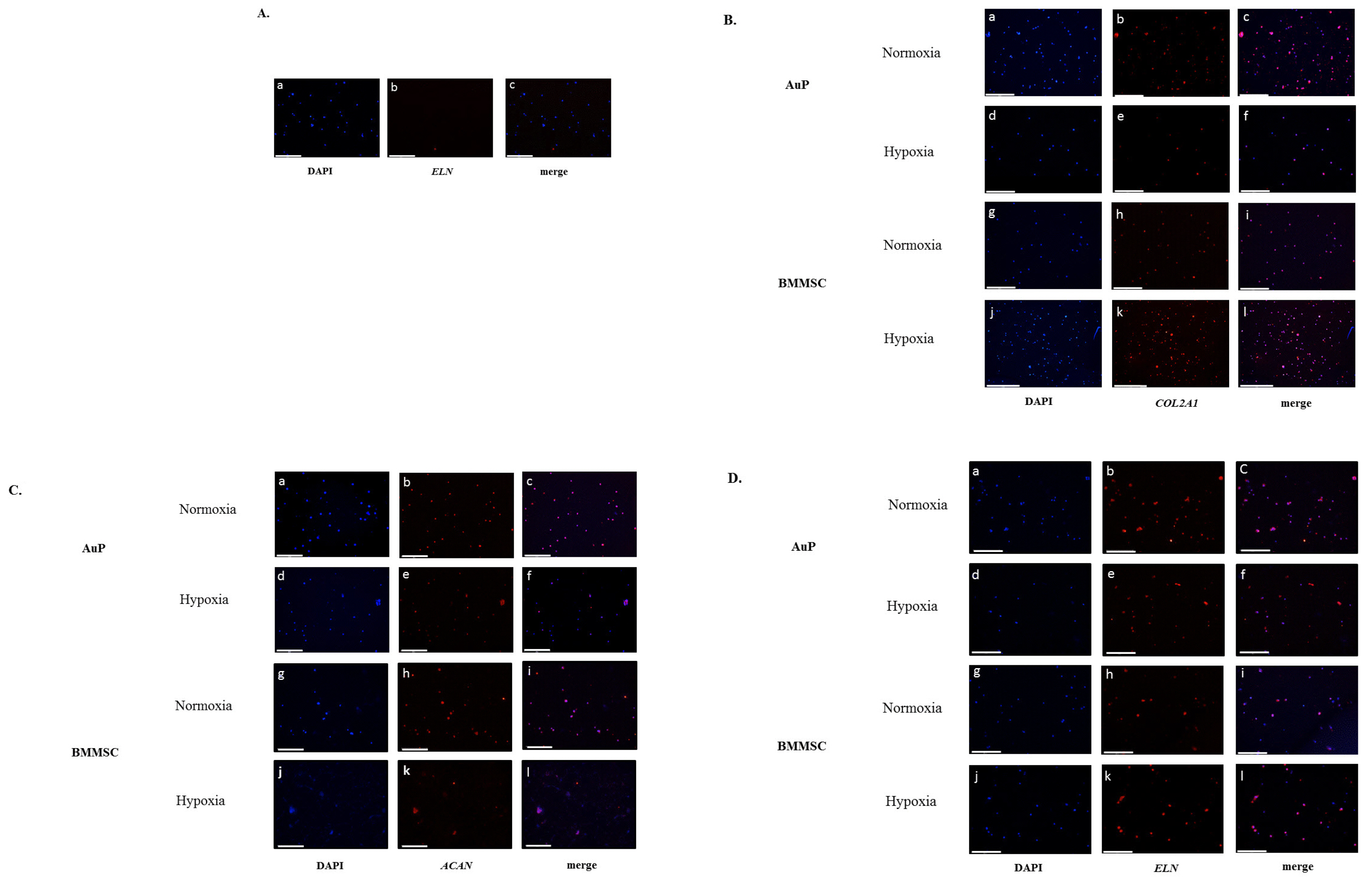
In this study, we investigated a natural scaffold (alginate) as tridimensional hydrogel seeded with progenitors from different origins such as bone marrow, perichondrium and dental pulp. In contact with the scaffold, these cells remained viable and were able to differentiate into chondrocytes when cultured in vitro. Quantitative and qualitative results show the presence of different chondrogenic markers as well as elastic ones for the purpose of ear cartilage, upon different culture conditions.
Conclusions
We confirmed that auricular perichondrial cells outperform other cells to produce chondrogenic tissue in normal oxygen levels and we report for the first time the effect of hypoxia on these cells. Our results provide updates for cartilage engineering for future clinical applications.
Keyword
Figure
Reference
-
References
1. Wernheden E, Krogerus C, Andersen PS, Hesselfeldt-Nielsen J. 2019; Congenital anomalies of the external ear. Ugeskr Laeger. 181:V05190300. Danish.2. Firmin F, Marchac A. 2011; A novel algorithm for autologous ear reconstruction. Semin Plast Surg. 25:257–264. DOI: 10.1055/s-0031-1288917. PMCID: PMC3312152.
Article3. Yang HC, Cho HH, Jo SY, Jang CH, Cho YB. 2015; Donor-site morbidity following minimally invasive costal cartilage harvest technique. Clin Exp Otorhinolaryngol. 8:13–19. DOI: 10.3342/ceo.2015.8.1.13. PMID: 25729490. PMCID: PMC4338086.
Article4. Li XS, Sun JJ. 2019; Regenerative medicine of tissue engineering: auricular cartilage regeneration and functional reconstruction. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 33:567–571. Chinese.5. Bernstein JL, Cohen BP, Lin A, Harper A, Bonassar LJ, Spector JA. 2018; Tissue engineering auricular cartilage using late passage human auricular chondrocytes. Ann Plast Surg. 80(4 Suppl 4):S168–S173. DOI: 10.1097/SAP.0000000000001400. PMID: 29537998. PMCID: PMC5910223.
Article6. Cohen BP, Hooper RC, Puetzer JL, Nordberg R, Asanbe O, Hernandez KA, Spector JA, Bonassar LJ. 2016; Long-term morphological and microarchitectural stability of tissue-engineered, patient-specific auricles in vivo. Tissue Eng Part A. 22:461–468. DOI: 10.1089/ten.tea.2015.0323. PMID: 26847742. PMCID: PMC4800266.
Article7. Kim H, Bae C, Kook YM, Koh WG, Lee K, Park MH. 2019; Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res Ther. 10:51. DOI: 10.1186/s13287-018-1130-8. PMID: 30732645. PMCID: PMC6367797.
Article8. Yang W, Chen Q, Xia R, Zhang Y, Shuai L, Lai J, You X, Jiang Y, Bie P, Zhang L, Zhang H, Bai L. 2018; A novel bioscaffold with naturally-occurring extracellular matrix promotes hepatocyte survival and vessel patency in mouse models of heterologous transplantation. Biomaterials. 177:52–66. DOI: 10.1016/j.biomaterials.2018.05.026. PMID: 29885586.
Article9. Gentile P, Ghione C, Ferreira AM, Crawford A, Hatton PV. 2017; Alginate-based hydrogels functionalised at the nanoscale using layer-by-layer assembly for potential cartilage repair. Biomater Sci. 5:1922–1931. DOI: 10.1039/C7BM00525C. PMID: 28752866.
Article10. Visscher DO, Gleadall A, Buskermolen JK, Burla F, Segal J, Koenderink GH, Helder MN, van Zuijlen PPM. 2019; Design and fabrication of a hybrid alginate hydrogel/poly(ε-caprolactone) mold for auricular cartilage reconstruction. J Biomed Mater Res B Appl Biomater. 107:1711–1721. DOI: 10.1002/jbm.b.34264. PMID: 30383916. PMCID: PMC6587956.
Article11. Duval E, Baugé C, Andriamanalijaona R, Bénateau H, Leclercq S, Dutoit S, Poulain L, Galéra P, Boumédiene K. 2012; Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials. 33:6042–6051. DOI: 10.1016/j.biomaterials.2012.04.061. PMID: 22677190.
Article12. Bae HC, Park HJ, Wang SY, Yang HR, Lee MC, Han HS. 2018; Hypoxic condition enhances chondrogenesis in synovium-derived mesenchymal stem cells. Biomater Res. 22:28. DOI: 10.1186/s40824-018-0134-x. PMID: 30275971. PMCID: PMC6158840.
Article13. Foyt DA, Taheem DK, Ferreira SA, Norman MDA, Petzold J, Jell G, Grigoriadis AE, Gentleman E. 2019; Hypoxia impacts human MSC response to substrate stiffness during chondrogenic differentiation. Acta Biomater. 89:73–83. DOI: 10.1016/j.actbio.2019.03.002. PMID: 30844569. PMCID: PMC6481516.
Article14. Schnabel M, Marlovits S, Eckhoff G, Fichtel I, Gotzen L, Vécsei V, Schlegel J. 2002; Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 10:62–70. DOI: 10.1053/joca.2001.0482. PMID: 11795984.
Article15. Khatab S, Leijs MJ, van Buul G, Haeck J, Kops N, Nieboer M, Bos PK, Verhaar JAN, Bernsen M, van Osch GJVM. 2020; MSC encapsulation in alginate microcapsules prolongs survival after intra-articular injection, a longitudinal in vivo cell and bead integrity tracking study. Cell Biol Toxicol. 36:553–570. DOI: 10.1007/s10565-020-09532-6. PMID: 32474743. PMCID: PMC7661423.
Article16. Kobayashi S, Takebe T, Zheng YW, Mizuno M, Yabuki Y, Maegawa J, Taniguchi H. 2011; Presence of cartilage stem/progenitor cells in adult mice auricular perichondrium. PLoS One. 6:e26393. DOI: 10.1371/journal.pone.0026393. PMID: 22039478. PMCID: PMC3198405.
Article17. Togo T, Utani A, Naitoh M, Ohta M, Tsuji Y, Morikawa N, Nakamura M, Suzuki S. 2006; Identification of cartilage progenitor cells in the adult ear perichondrium: utilization for cartilage reconstruction. Lab Invest. 86:445–457. DOI: 10.1038/labinvest.3700409. PMID: 16625212.
Article18. Xue K, Zhang X, Qi L, Zhou J, Liu K. 2016; Isolation, identification, and comparison of cartilage stem progenitor/cells from auricular cartilage and perichondrium. Am J Transl Res. 8:732–741.19. Derks M, Sturm T, Haverich A, Hilfiker A. 2013; Isolation and chondrogenic differentiation of porcine perichondrial progenitor cells for the purpose of cartilage tissue engineering. Cells Tissues Organs. 198:179–189. DOI: 10.1159/000354897. PMID: 24157487.
Article20. Kagimoto S, Takebe T, Kobayashi S, Yabuki Y, Hori A, Hirotomi K, Mikami T, Uemura T, Maegawa J, Taniguchi H. 2016; Autotransplantation of monkey ear perichondrium-deri-ved progenitor cells for cartilage reconstruction. Cell Transplant. 25:951–962. DOI: 10.3727/096368916X690917. PMID: 26884211.
Article21. Zhang Y, Feng G, Xu G, Qi Y. 2019; Microporous acellular extracellular matrix combined with adipose-derived stem cell sheets as a promising tissue patch promoting articular cartilage regeneration and interface integration. Cytotherapy. 21:856–869. DOI: 10.1016/j.jcyt.2019.02.005. PMID: 31196819.
Article22. Nemeth CL, Janebodin K, Yuan AE, Dennis JE, Reyes M, Kim DH. 2014; Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng Part A. 20:2817–2829. DOI: 10.1089/ten.tea.2013.0614. PMID: 24749806. PMCID: PMC4229712.
Article23. Talaat W, Aryal Ac S, Al Kawas S, Samsudin ABR, Kandile NG, Harding DRK, Ghoneim MM, Zeiada W, Jagal J, Aboelnaga A, Haider M. 2020; Nanoscale thermosensitive hydrogel scaffolds promote the chondrogenic differentiation of dental pulp stem and progenitor cells: a minimally invasive approach for cartilage regeneration. Int J Nanomedicine. 15:7775–7789. DOI: 10.2147/IJN.S274418. PMID: 33116500. PMCID: PMC7567564.24. Fernandes TL, Shimomura K, Asperti A, Pinheiro CCG, Caetano HVA, Oliveira CRGCM, Nakamura N, Hernandez AJ, Bueno DF. 2018; Development of a novel large animal model to evaluate human dental pulp stem cells for articular cartilage treatment. Stem Cell Rev Rep. 14:734–743. DOI: 10.1007/s12015-018-9820-2. PMID: 29728886. PMCID: PMC6132738.
Article25. Longoni A, Utomo L, van Hooijdonk IE, Bittermann GK, Vetter VC, Kruijt Spanjer EC, Ross J, Rosenberg AJ, Gawlitta D. 2020; The chondrogenic differentiation potential of dental pulp stem cells. Eur Cell Mater. 39:121–135. DOI: 10.22203/eCM.v039a08. PMID: 32083715.
Article26. Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, Asnaghi AM, Baumhoer D, Bieri O, Kretzschmar M, Pagenstert G, Haug M, Schaefer DJ, Martin I, Jakob M. 2016; Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 388:1985–1994. DOI: 10.1016/S0140-6736(16)31658-0. PMID: 27789021.
Article27. Li T, Chen S, Pei M. 2020; Contribution of neural crest-derived stem cells and nasal chondrocytes to articular cartilage regeneration. Cell Mol Life Sci. 77:4847–4859. DOI: 10.1007/s00018-020-03567-y. PMID: 32504256. PMCID: PMC9150440.
Article28. do Amaral RJ, Pedrosa Cda S, Kochem MC, Silva KR, Aniceto M, Claudio-da-Silva C, Borojevic R, Baptista LS. 2012; Isolation of human nasoseptal chondrogenic cells: a promise for cartilage engineering. Stem Cell Res. 8:292–299. DOI: 10.1016/j.scr.2011.09.006. PMID: 22099383.
Article29. Asnaghi MA, Power L, Barbero A, Haug M, Köppl R, Wendt D, Martin I. 2020; Biomarker signatures of quality for engineering nasal chondrocyte-derived cartilage. Front Bioeng Biotechnol. 8:283. DOI: 10.3389/fbioe.2020.00283. PMID: 32318561. PMCID: PMC7154140.
Article30. Ma T, Grayson WL, Fröhlich M, Vunjak-Novakovic G. 2009; Hypoxia and stem cell-based engineering of mesenchymal tissues. Biotechnol Prog. 25:32–42. DOI: 10.1002/btpr.128. PMID: 19198002. PMCID: PMC2771546.
Article31. Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. 2005; Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 37:313–322. DOI: 10.1016/j.bone.2005.04.040. PMID: 16023419.
Article32. Chen C, Huang K, Zhu J, Bi Y, Wang L, Jiang J, Zhu T, Yan X, Zhao J. 2020; A novel elastic and controlled-release poly (ether-ester-urethane)urea scaffold for cartilage regeneration. J Mater Chem B. 8:4106–4121. DOI: 10.1039/C9TB02754H. PMID: 32253395.
Article33. Weizel A, Distler T, Schneidereit D, Friedrich O, Bräuer L, Paulsen F, Detsch R, Boccaccini AR, Budday S, Seitz H. 2020; Complex mechanical behavior of human articular cartilage and hydrogels for cartilage repair. Acta Biomater. 118:113–128. DOI: 10.1016/j.actbio.2020.10.025. PMID: 33080391.
Article34. Andriamanalijaona R, Duval E, Raoudi M, Lecourt S, Vilquin JT, Marolleau JP, Pujol JP, Galera P, Boumediene K. 2008; Differentiation potential of human muscle-derived cells towards chondrogenic phenotype in alginate beads culture. Osteoarthritis Cartilage. 16:1509–1518. DOI: 10.1016/j.joca.2008.04.018. PMID: 18554936.
Article35. Cai X, Lin Y, Ou G, Luo E, Man Y, Yuan Q, Gong P. 2007; Ectopic osteogenesis and chondrogenesis of bone marrow stromal stem cells in alginate system. Cell Biol Int. 31:776–783. DOI: 10.1016/j.cellbi.2007.01.011. PMID: 17324591.
Article36. Wu YN, Yang Z, Hui JH, Ouyang HW, Lee EH. 2007; Cartilaginous ECM component-modification of the micro-bead culture system for chondrogenic differentiation of mesenchymal stem cells. Biomaterials. 28:4056–4067. DOI: 10.1016/j.biomaterials.2007.05.039. PMID: 17590431.
Article37. Estes BT, Wu AW, Guilak F. 2006; Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 54:1222–1232. DOI: 10.1002/art.21779. PMID: 16572454.
Article38. Majumdar MK, Banks V, Peluso DP, Morris EA. 2000; Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol. 185:98–106. DOI: 10.1002/1097-4652(200010)185:1<98::AID-JCP9>3.0.CO;2-1. PMID: 10942523.
Article39. Otto IA, Levato R, Webb WR, Khan IM, Breugem CC, Malda J. 2018; Progenitor cells in auricular cartilage demonstrate cartilage-forming capacity in 3D hydrogel culture. Eur Cell Mater. 35:132–150. DOI: 10.22203/eCM.v035a10. PMID: 29485180.
Article40. Hellingman CA, Verwiel ET, Slagt I, Koevoet W, Poublon RM, Nolst-Trenité GJ, Baatenburg de Jong RJ, Jahr H, van Osch GJ. 2011; Differences in cartilage-forming capacity of expanded human chondrocytes from ear and nose and their gene expression profiles. Cell Transplant. 20:925–940. DOI: 10.3727/096368910X539119. PMID: 21054934.
Article41. Fernandes TL, Cortez de SantAnna JP, Frisene I, Gazarini JP, Gomes Pinheiro CC, Gomoll AH, Lattermann C, Hernandez AJ, Franco Bueno D. 2020; Systematic review of human dental pulp stem cells for cartilage regeneration. Tissue Eng Part B Rev. 26:1–12. DOI: 10.1089/ten.teb.2019.0140. PMID: 31744404.
Article42. Mata M, Milian L, Oliver M, Zurriaga J, Sancho-Tello M, de Llano JJM, Carda C. 2017; In vivo articular cartilage regene-ration using human dental pulp stem cells cultured in an alginate scaffold: a preliminary study. Stem Cells Int. 2017:8309256. DOI: 10.1155/2017/8309256. PMID: 28951745. PMCID: PMC5603743.43. Westin CB, Trinca RB, Zuliani C, Coimbra IB, Moraes ÂM. 2017; Differentiation of dental pulp stem cells into chondrocytes upon culture on porous chitosan-xanthan scaffolds in the presence of kartogenin. Mater Sci Eng C Mater Biol Appl. 80:594–602. DOI: 10.1016/j.msec.2017.07.005. PMID: 28866206.
Article44. Novais A, Lesieur J, Sadoine J, Slimani L, Baroukh B, Saubaméa B, Schmitt A, Vital S, Poliard A, Hélary C, Rochefort GY, Chaussain C, Gorin C. 2019; Priming dental pulp stem cells from human exfoliated deciduous teeth with fibroblast growth factor-2 enhances mineralization within tissue-engineered constructs implanted in craniofacial bone defects. Stem Cells Transl Med. 8:844–857. DOI: 10.1002/sctm.18-0182. PMID: 31016898. PMCID: PMC6646701.
Article45. Wang F, Hu Y, He D, Zhou G, Yang X, Ellis E 3rd. 2017; Regeneration of subcutaneous tissue-engineered mandibular condyle in nude mice. J Craniomaxillofac Surg. 45:855–861. DOI: 10.1016/j.jcms.2017.03.017. PMID: 28462782.
Article46. Ito K, Matsuoka K, Matsuzaka K, Morinaga K, Inoue T. 2015; Hypoxic condition promotes differentiation and mineralization of dental pulp cells in vivo. Int Endod J. 48:115–123. DOI: 10.1111/iej.12288. PMID: 24661255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor
- Differentiation of neuroepithelial progenitor cells implanted into newborn rat brain striatum
- Mesengenic Differentiation: Comparison of Human and Rat Bone Marrow Mesenchymal Stem Cells
- Stem Cells for the Regeneration of Tendon and Ligament: A Perspective
- The Role of Hypoxia in Brain Tumor Immune Responses