Diabetes Metab J.
2023 Jul;47(4):523-534. 10.4093/dmj.2022.0096.
Two-Year Changes in Diabetic Kidney Disease Phenotype and the Risk of Heart Failure: A Nationwide Population-Based Study in Korea
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Korea
- 2Department of Biomedicine & Health Science, The Catholic University of Korea, Seoul, Korea
- 3Department of Statistics and Actuarial Science, Soongsil University, Seoul, Korea
- KMID: 2544731
- DOI: http://doi.org/10.4093/dmj.2022.0096
Abstract
- Background
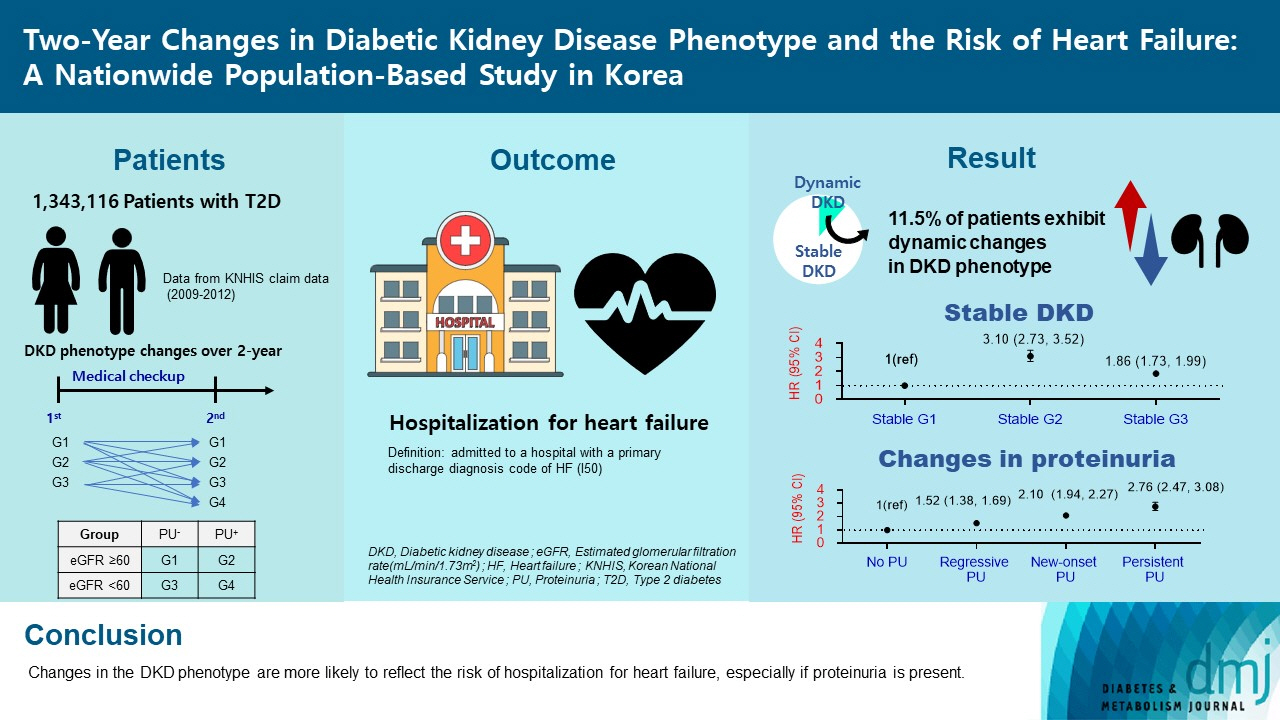
Diabetic kidney disease (DKD) is a risk factor for hospitalization for heart failure (HHF). DKD could be classified into four phenotypes by estimated glomerular filtration rate (eGFR, normal vs. low) and proteinuria (PU, negative vs. positive). Also, the phenotype often changes dynamically. This study examined HHF risk according to the DKD phenotype changes across 2-year assessments.
Methods
The study included 1,343,116 patients with type 2 diabetes mellitus (T2DM) from the Korean National Health Insurance Service database after excluding a very high-risk phenotype (eGFR <30 mL/min/1.73 m2) at baseline, who underwent two cycles of medical checkups between 2009 and 2014. From the baseline and 2-year eGFR and PU results, participants were divided into 10 DKD phenotypic change categories.
Results
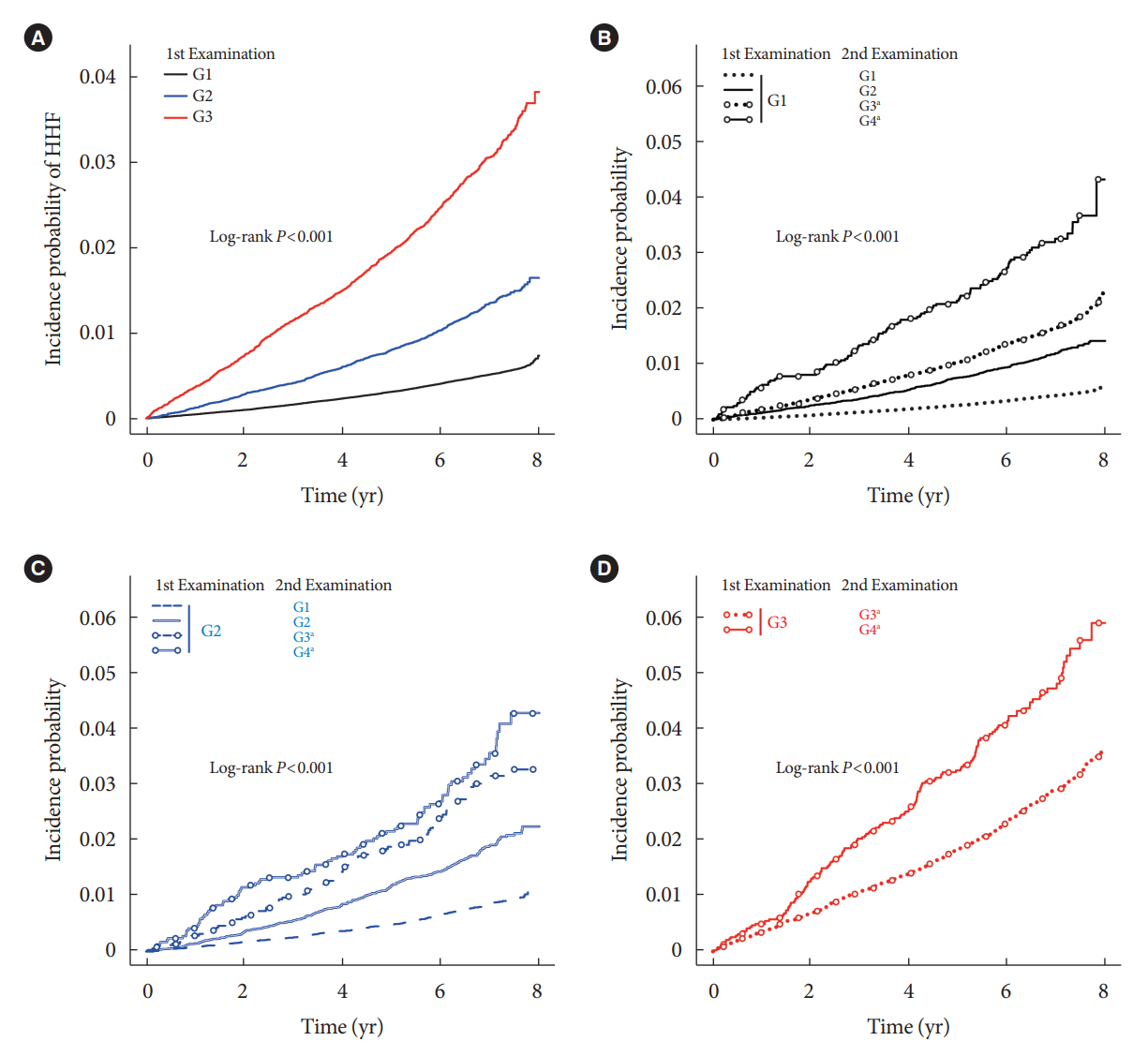
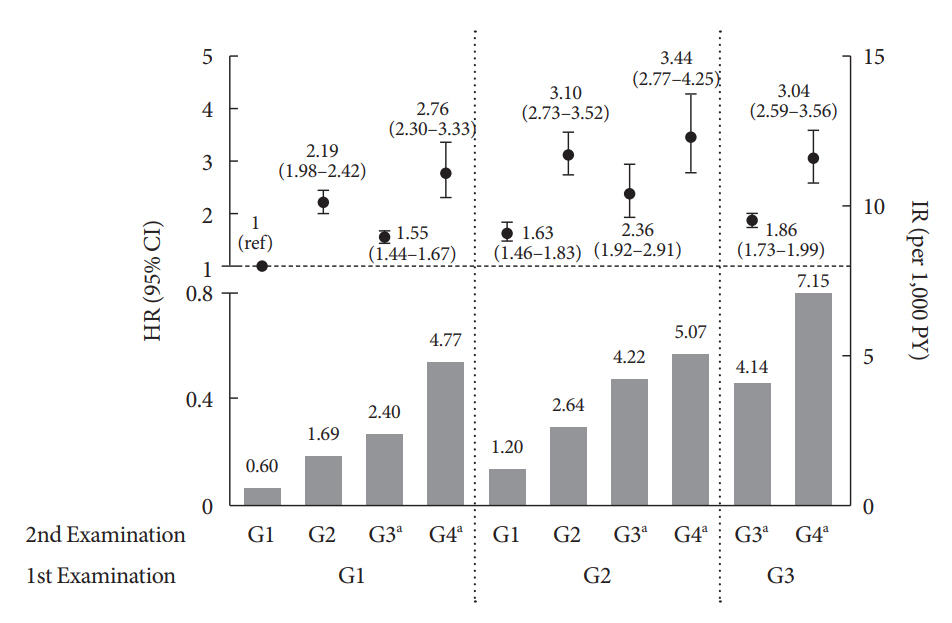
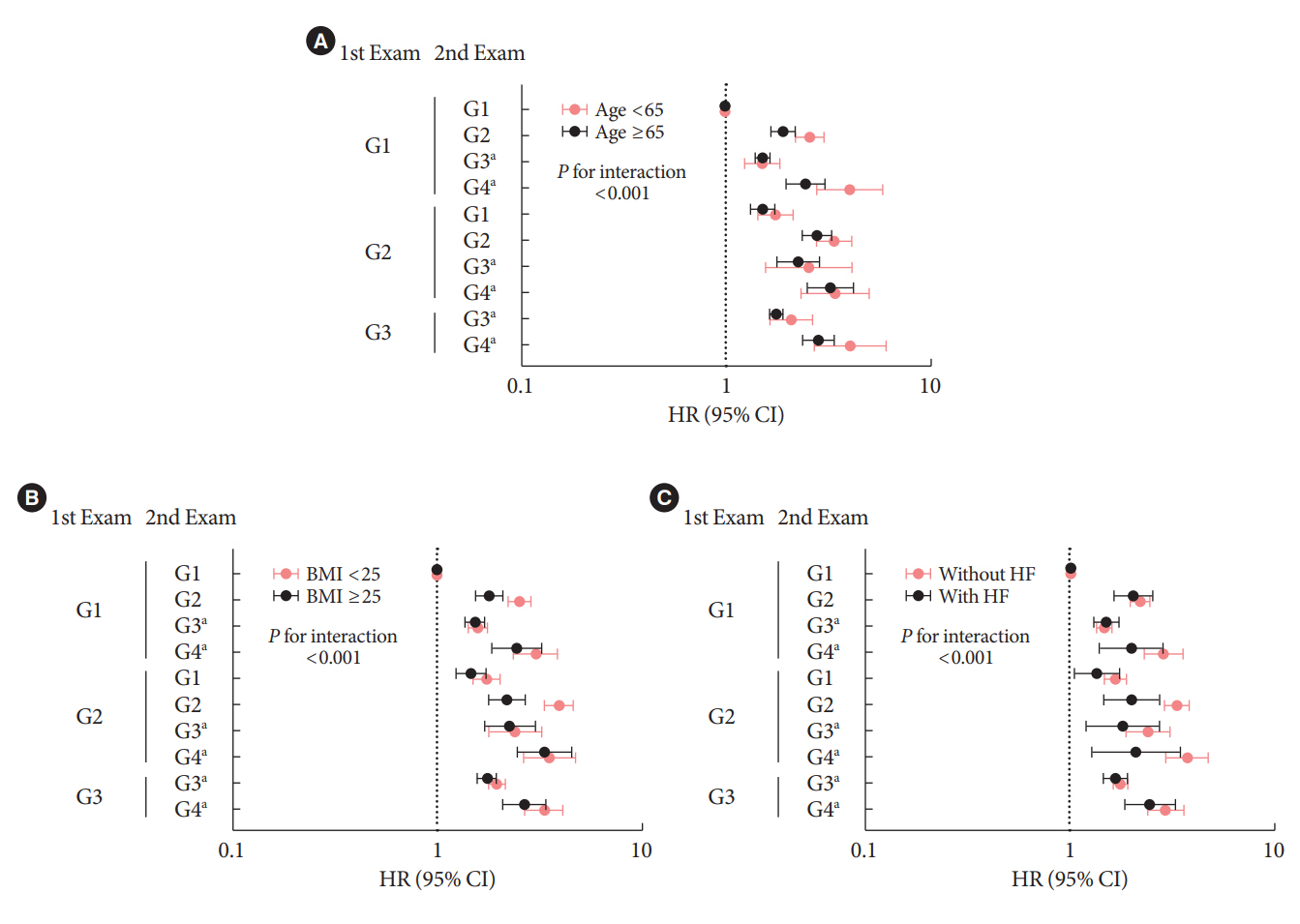
During an average of 6.5 years of follow-up, 7,874 subjects developed HHF. The cumulative incidence of HHF from index date was highest in the eGFRlowPU– phenotype, followed by eGFRnorPU+ and eGFRnorPU–. Changes in DKD phenotype differently affect HHF risk. When the persistent eGFRnorPU– category was the reference, hazard ratios for HHF were 3.10 (95% confidence interval [CI], 2.73 to 3.52) in persistent eGFRnorPU+ and 1.86 (95% CI, 1.73 to 1.99) in persistent eGFRlowPU–. Among altered phenotypes, the category converted to eGFRlowPU+ showed the highest risk. In the normal eGFR category at the second examination, those who converted from PU– to PU+ showed a higher risk of HHF than those who converted from PU+ to PU–.
Conclusion
Changes in DKD phenotype, particularly with the presence of PU, are more likely to reflect the risk of HHF, compared with DKD phenotype based on a single time point in patients with T2DM.
Figure
Reference
-
1. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020; 395:709–33.2. Birkeland KI, Bodegard J, Eriksson JW, Norhammar A, Haller H, Linssen GC, et al. Heart failure and chronic kidney disease manifestation and mortality risk associations in type 2 diabetes: a large multinational cohort study. Diabetes Obes Metab. 2020; 22:1607–18.
Article3. Greene SJ, Butler J. Primary prevention of heart failure in patients with type 2 diabetes mellitus. Circulation. 2019; 139:152–4.
Article4. Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med. 2014; 370:1514–23.
Article5. American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. 11. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S175–84.6. Oshima M, Shimizu M, Yamanouchi M, Toyama T, Hara A, Furuichi K, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol. 2021; 17:740–50.
Article7. Yokoyama H, Araki SI, Kawai K, Yamazaki K, Shirabe SI, Sugimoto H, et al. The prognosis of patients with type 2 diabetes and nonalbuminuric diabetic kidney disease is not always poor: implication of the effects of coexisting macrovascular complications (JDDM 54). Diabetes Care. 2020; 43:1102–10.
Article8. Buyadaa O, Magliano DJ, Salim A, Koye DN, Shaw JE. Risk of rapid kidney function decline, all-cause mortality, and major cardiovascular events in nonalbuminuric chronic kidney disease in type 2 diabetes. Diabetes Care. 2020; 43:122–9.
Article9. Lee SE, Yoo J, Kim KA, Han K, Choi HS. Hip fracture risk according to diabetic kidney disease phenotype in a Korean population. Endocrinol Metab (Seoul). 2022; 37:148–58.
Article10. Korean National Health Insurance Service: National Health Insurance Data Sharing Service. Available from: https://nhiss.nhis.or.kr/bd/ab/bdaba000eng.do (cited 2023 Feb 10).11. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020; 98(4S):S1–115.12. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–70.
Article13. Avery CL, Loehr LR, Baggett C, Chang PP, Kucharska-Newton AM, Matsushita K, et al. The population burden of heart failure attributable to modifiable risk factors: the ARIC (Atherosclerosis Risk in Communities) study. J Am Coll Cardiol. 2012; 60:1640–6.14. Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018; 20:853–72.
Article15. Kievit RF, Gohar A, Hoes AW, Bots ML, van Riet EE, van Mourik Y, et al. Efficient selective screening for heart failure in elderly men and women from the community: a diagnostic individual participant data meta-analysis. Eur J Prev Cardiol. 2018; 25:437–46.
Article16. van Giessen A, Boonman-de Winter LJ, Rutten FH, Cramer MJ, Landman MJ, Liem AH, et al. Cost-effectiveness of screening strategies to detect heart failure in patients with type 2 diabetes. Cardiovasc Diabetol. 2016; 15:48.
Article17. Park JI, Baek H, Jung HH. Prevalence of chronic kidney disease in Korea: the Korean National Health and Nutritional Examination Survey 2011-2013. J Korean Med Sci. 2016; 31:915–23.
Article18. Matsushita K, Ballew SH, Coresh J. Cardiovascular risk prediction in people with chronic kidney disease. Curr Opin Nephrol Hypertens. 2016; 25:518–23.
Article19. Ballew SH, Matsushita K. Cardiovascular risk prediction in CKD. Semin Nephrol. 2018; 38:208–16.
Article20. Berg DD, Wiviott SD, Scirica BM, Gurmu Y, Mosenzon O, Murphy SA, et al. Heart failure risk stratification and efficacy of sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes mellitus. Circulation. 2019; 140:1569–77.
Article21. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. 2021; 44(Suppl 1):S151–67.22. Herrington WG, Preiss D, Haynes R, von Eynatten M, Staplin N, Hauske SJ, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPAKIDNEY study. Clin Kidney J. 2018; 11:749–61.
Article23. Wang A, Sun Y, Liu X, Su Z, Li J, Luo Y, et al. Changes in proteinuria and the risk of myocardial infarction in people with diabetes or pre-diabetes: a prospective cohort study. Cardiovasc Diabetol. 2017; 16:104.
Article24. Park JI, Baek H, Kim BR, Jung HH. Comparison of urine dipstick and albumin:creatinine ratio for chronic kidney disease screening: a population-based study. PLoS One. 2017; 12:e0171106.
Article25. Dunlay SM, Givertz MM, Aguilar D, Allen LA, Chan M, Desai AS, et al. Type 2 diabetes mellitus and heart failure: a scientific statement from the American Heart Association and the Heart Failure Society of America. This statement does not represent an update of the 2017 ACC/AHA/HFSA heart failure guideline update. Circulation. 2019; 140:e294–324.
Article26. Folkerts K, Petruski-Ivleva N, Comerford E, Blankenburg M, Evers T, Gay A, et al. Adherence to chronic kidney disease screening guidelines among patients with type 2 diabetes in a US Administrative Claims Database. Mayo Clin Proc. 2021; 96:975–86.
Article27. Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009; 54:205–26.
Article28. Choi HM, Park MS, Youn JC. Update on heart failure management and future directions. Korean J Intern Med. 2019; 34:11–43.
Article29. Thomas B. The global burden of diabetic kidney disease: time trends and gender gaps. Curr Diab Rep. 2019; 19:18.
Article30. van der Meer P, Gaggin HK, Dec GW. ACC/AHA versus ESC guidelines on heart failure: JACC guideline comparison. J Am Coll Cardiol. 2019; 73:2756–68.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and test for diabetic kidney disease
- Diabetes and Heart Failure
- Albuminuria, estimated glomerular filtration rate, and traditional predictors for composite cardiovascular and kidney outcome: a population-based cohort study in Korea
- Weight change and risk of depression in patients with diabetic kidney disease: a nationwide population-based study
- Echocardiographic Diagnosis of Diabetic Cardiomyopathy