J Korean Med Sci.
2023 Jul;38(27):e211. 10.3346/jkms.2023.38.e211.
Effectiveness of Paxlovid, an Oral Antiviral Drug, Against the Omicron BA.5 Variant in Korea: Severe Progression and Death Between July and November 2022
- Affiliations
-
- 1Patient Management Team, Central Disease Control Headquarters for COVID-19, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 2Division of Emerging Infectious Disease, Bureau of Infectious Disease Risk Response, Korea Disease Control and Prevention Agency, Cheongju, Korea
- KMID: 2544413
- DOI: http://doi.org/10.3346/jkms.2023.38.e211
Abstract
- Background
Paxlovid is an oral antiviral drug that received emergency use authorization in South Korea for the treatment of patients with mild-to-moderate coronavirus disease 2019 (COVID-19) on January 14, 2022. Since the onset of the severe acute respiratory syndrome coronavirus 2 pandemic, the virus has continued to evolve. The emergence of new variants has raised concerns about possible reductions in the effectiveness of vaccines and drugs. The effectiveness of Paxlovid in patients infected with the omicron variant and subvariants has not yet been determined. This study assessed the effectiveness of Paxlovid at reducing the risk of severe/critical illness or death and death in patients with mild-to-moderate COVID-19 caused by omicron subvariant BA.5.
Methods
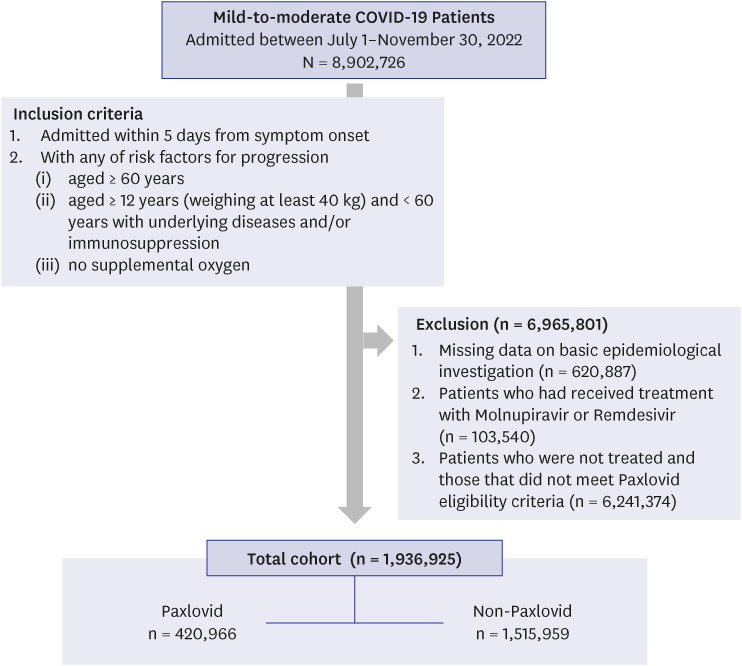
In this nationwide retrospective cohort study, data on 8,902,726 patients were collected from four sources (the Drug Utilization Review database, COVID-19 Patient Information Management System, confirmed patient information, and basic epidemiological investigation data) between July 1 and November 30, 2022. Multivariable logistic regression analysis was conducted, with adjustment for age, sex, severe acute respiratory syndrome coronavirus 2 immunity (vaccination), and comorbidities.
Results
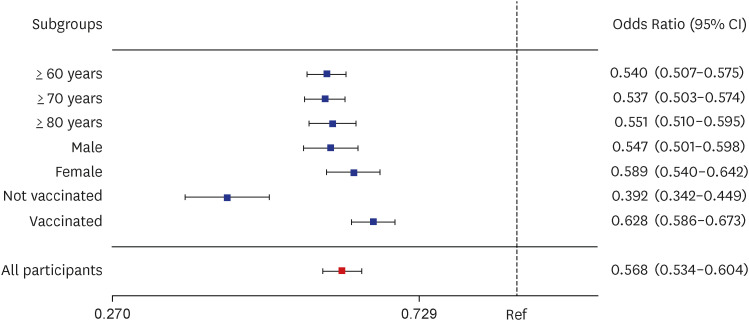
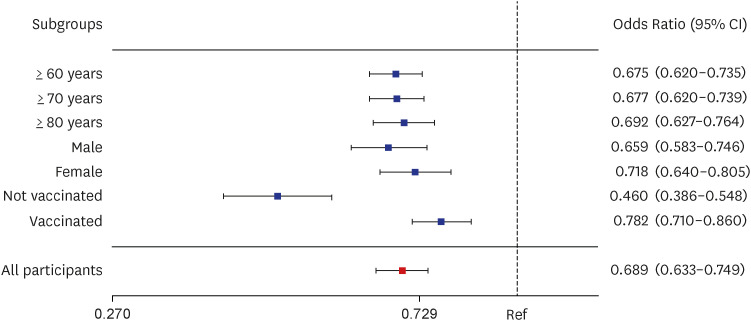
A total of 1,936,925 patients with COVID-19 were included in the analysis, including 420,996 patients treated with Paxlovid, and 1,515,959 patients not treated with Paxlovid. Paxlovid treatment in patients aged ≥ 60 years of age was associated with significantly reduced risk of severe/critical illness or death (46.0%), and death rate (32.5%), and its effectiveness was high, regardless of vaccination status.
Conclusion
Paxlovid is effective at reducing the risk of death due to COVID-19 in patients with omicron BA.5 infection, especially in older patients, regardless of vaccination status. This suggests that older patients with COVID-19-related symptoms should be administered Paxlovid, regardless of their vaccination status, to reduce severity and risk of death.
Keyword
Figure
Reference
-
1. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579(7798):270–273. PMID: 32015507.2. Zhu H, Wei L, Niu P. The novel coronavirus outbreak in Wuhan, China. Glob Health Res Policy. 2020; 5(1):6. PMID: 32226823.3. World Health Organization. Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Updated 2021. Accessed November 26, 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern .4. Zheng JX, Lv S, Tian LG, Guo ZY, Zheng PY, Chen YL, et al. The rapid and efficient strategy for SARS-CoV-2 Omicron transmission control: analysis of outbreaks at the city level. Infect Dis Poverty. 2022; 11(1):114. PMID: 36434701.5. Baker JM, Nakayama JY, O’Hegarty M, McGowan A, Teran RA, Bart SM, et al. SARS-CoV-2 B.1.1.529 (omicron) variant transmission within households – Four U.S. jurisdictions, November 2021–February 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(9):341–346. PMID: 35238860.6. Ito K, Piantham C, Nishiura H. Relative instantaneous reproduction number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark. J Med Virol. 2022; 94(5):2265–2268. PMID: 34967453.7. Korea Centers for Disease Control & Prevention. Emergency implementation to block inflow into Korea and prevent transmission of a total of 5 confirmed cases of omicron mutant virus in Korea, press reference data. Updated 2021. Accessed March 20, 2022. http://ncov.mohw.go.kr/upload/viewer/skin/doc.html?fn=1638364982419_20211201222302.pdf&rs=/upload/viewer/result/202205/ .8. Korea Centers for Disease Control & Prevention. Characteristic analysis of Omicron variations, press reference data. Updated 2022. Accessed January 24, 2022. http://ncov.mohw.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6313&contSeq=6313&board_id=312&gubun=BDJ .9. Kim EY, Choe YJ, Park H, Jeong H, Chung JH, Yu J, et al. Community transmission of SARS-CoV-2 omicron variant, South Korea, 2021. Emerg Infect Dis. 2022; 28(4):898–900. PMID: 35171760.10. Korea Disease Control and Prevention Agency. Updated 2022. Accessed September 13, 2022. https://www.kdca.go.kr/board/board.es?mid=a20501000000&bid=0015&list_no=720694&cg_code=&act=view&nPage=18 .11. Sun K, Tempia S, Kleynhans J, von Gottberg A, McMorrow ML, Wolter N, et al. SARS-CoV-2 transmission, persistence of immunity, and estimates of Omicron’s impact in South African population cohorts. Sci Transl Med. 2022; 14(659):eabo7081. PMID: 35638937.12. Ward IL, Bermingham C, Ayoubkhani D, Gethings OJ, Pouwels KB, Yates T, et al. Risk of covid-19 related deaths for SARS-CoV-2 omicron (B.1.1.529) compared with delta (B.1.617.2): retrospective cohort study. BMJ. 2022; 378:e070695. PMID: 35918098.13. Modes ME, Directo MP, Melgar M, Johnson LR, Yang H, Chaudhary P, et al. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed SARS-CoV-2 infection during periods of B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant predominance - One Hospital, California, July 15-September 23, 2021, and December 21, 2021-January 27, 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(6):217–223. PMID: 35143466.14. Chavda VP, Apostolopoulos V. Omicron variant (B.1.1.529) of SARS-CoV-2: Threat for the elderly? Maturitas. 2022; 158:78–81. PMID: 35241241.15. Ying-Hao P, Yuan-Yuan G, Hai-Dong Z, Qiu-Hua C, Xue-Ran G, Hai-Qi Z, et al. Clinical characteristics and analysis of risk factors for disease progression of patients with SARS-CoV-2 Omicron variant infection: a retrospective study of 25207 cases in a Fangcang hospital. Front Cell Infect Microbiol. 2022; 12:1009894. PMID: 36389157.16. Hammond J, Leister-Tebbe H, Gardner A, Abreu P, Bao W, Wisemandle W, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022; 386(15):1397–1408. PMID: 35172054.17. Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, et al. Effectiveness of Paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023; 76(3):e342–e349. PMID: 35653428.18. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020; 30(3):269–271. PMID: 32020029.19. Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021; 374(6575):1586–1593. PMID: 34726479.20. Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med. 2020; 12(541):eabb5883. PMID: 32253226.21. Weng C, Xie R, Han G, Yuan Y, Li S, Wang C, et al. Safety and efficacy of Paxlovid against omicron variants of coronavirus disease 2019 in elderly patients. Infect Dis Ther. 2023; 12(2):649–662. PMID: 36696068.22. Sun F, Lin Y, Wang X, Gao Y, Ye S. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect Dis. 2022; 22(9):1279.23. Mahase E. Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021; 375(2713):n2713. PMID: 34750163.24. Devresse A, Sébastien Briol , De Greef J, Lemaitre F, Boland L, Haufroid V, et al. Safety, efficacy, and relapse of Nirmatrelvir-Ritonavir in kidney transplant recipients infected with SARS-CoV-2. Kidney Int Rep. 2022; 7(11):2356–2363. PMID: 36060621.25. Shah MM, Joyce B, Plumb ID, Sahakian S, Feldstein LR, Barkley E, et al. Paxlovid associated with decreased hospitalization rate among adults with COVID-19 - United States, April-September 2022. Am J Transplant. 2023; 23(1):150–155. PMID: 36695616.26. Korea Centers for Disease Control & Prevention. COVID-19 treatment guidelines. Guideline data. Updated 2022. Accessed May 13, 2022. https://www.kdca.go.kr/board/board.es?mid=a20507020000&bid=0019 .27. World Health Organization. Clinical management of COVID-19; 2020: Living guideline. Updated 2022. Accessed January 30, 2023. https://www.who.int/publications/i/item/clinical-management-of-covid-19 .28. Food and Drug Administration. Fact sheet for healthcare providers: emergency use authorization for Paxlovid. Updated 2021. Accessed January 18, 2022. https://www.fda.gov/media/155050/download .29. Korea Disease Control and Prevention Agency. Domestic COVID-19 mutant virus detection rate in South Korea. Updated 2022. Accessed September 1, 2022. http://www.kdca.go.kr/contents.es?mid=a20107040000 .30. World Health Organization. Interim Statement on COVID-19 vaccines in the context of the circulation of the Omicron SARS-CoV-2 Variant from the WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VOC). Updated 2022. Accessed December 2, 2022. https://www.who.int/news/item/11-01-2022-interim-statement-on-covid-19-vaccines-in-the-context-of-the-circulation-of-the-omicron-sars-cov-2-variant-from-the-who-technical-advisory-group-on-covid-19-vaccine-composition .31. Nham E, Song JY, Noh JY, Cheong HJ, Kim WJ. COVID-19 vaccination in Korea: past, present, and the way forward. J Korean Med Sci. 2022; 37(47):e351. PMID: 36472087.32. Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021; 384(15):1412–1423. PMID: 33626250.33. Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021; 397(10287):1819–1829. PMID: 33964222.34. Tenforde MW, Patel MM, Ginde AA, Douin DJ, Talbot HK, Casey JD, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis. 2022; 74(9):1515–1524. PMID: 34358310.35. Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021; 326(8):728–735. PMID: 34251417.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effectiveness of Paxlovid treatment in long-term care facilities in South Korea during the outbreak of the Omicron variant of SARS-CoV-2
- Effect of Paxlovid in COVID-19 treatment during the periods of SARS-CoV-2 Omicron BA.5 and BN.1 subvariant dominance in the Republic of Korea: a retrospective cohort study
- SARS-CoV-2 Omicron Variant of Concern: Everything You Wanted to Know about Omicron but Were Afraid to Ask
- Risk Factors Related to COVID-19 Reinfection and Fatality During the Omicron (BA.1/BA.2) Period in Korea
- Multi-Faceted Analysis of COVID-19 Epidemic in Korea Considering Omicron Variant: Mathematical Modeling-Based Study