Cancer Res Treat.
2023 Jul;55(3):948-955. 10.4143/crt.2023.290.
The Oncologic Implications of Tumor Multiplicity in Intrahepatic Cholangiocarcinoma: Its Prognostic Value Might Be Underestimated
- Affiliations
-
- 1Division of Hepatobiliary-Pancreatic Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Division of Transplant Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2544175
- DOI: http://doi.org/10.4143/crt.2023.290
Abstract
- Purpose
In the latest staging system of the American Joint Committee on Cancer for intrahepatic cholangiocarcinoma (IHCCC), solitary tumors with vascular invasion and multiple tumors are grouped together as T2. However, recent studies report that multifocal IHCCC has a worse prognosis than a single lesion. This study aimed to investigate the risk factors for IHCCC and explore the prognostic significance of multiplicity after surgical resection.
Materials and Methods
A total of 257 patients underwent surgery for IHCCC from 2010 to 2019 and the clinicopathological data were retrospectively reviewed. Risk factor analysis was performed to identify variables associated with survival after resection. Survival outcomes were compared between patients with solitary and multiple tumors.
Results
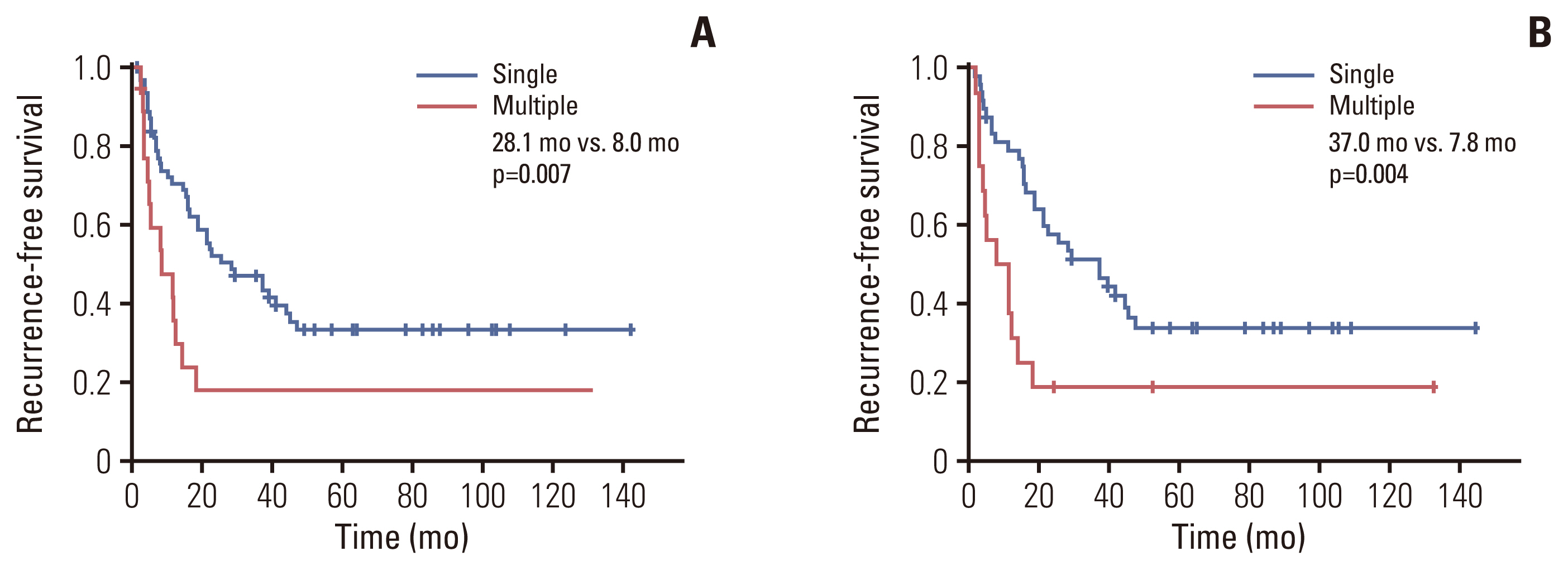
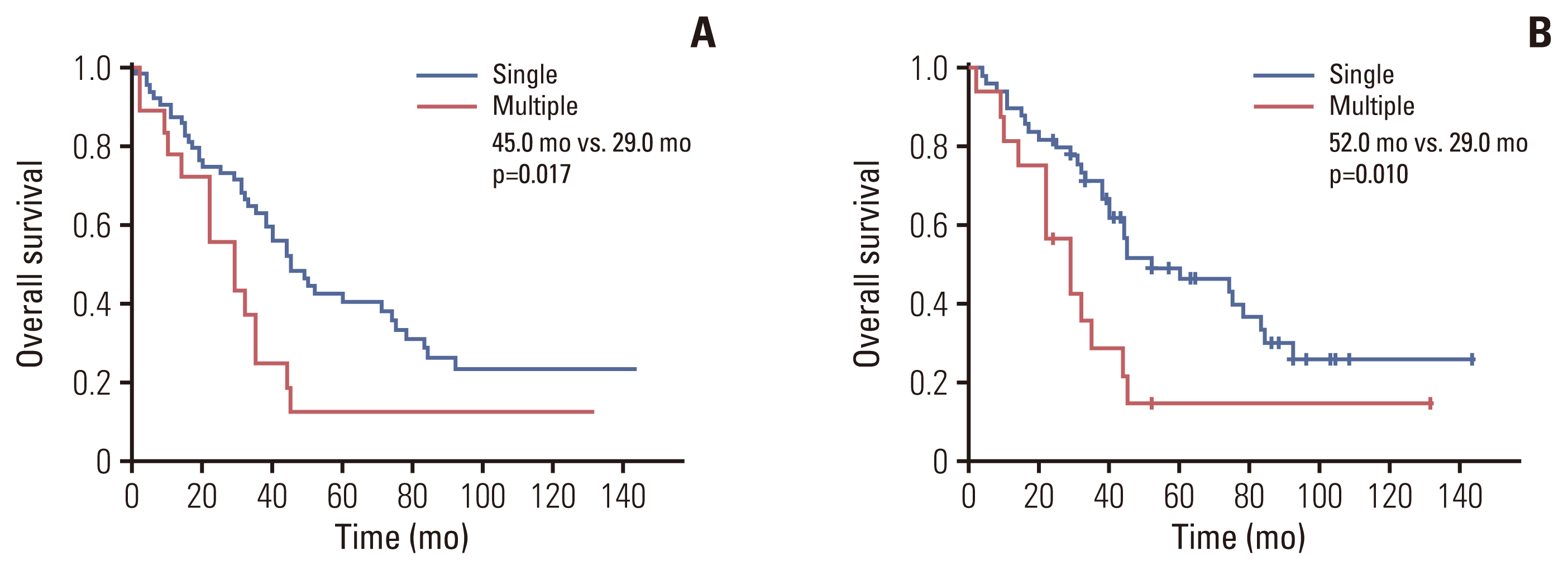
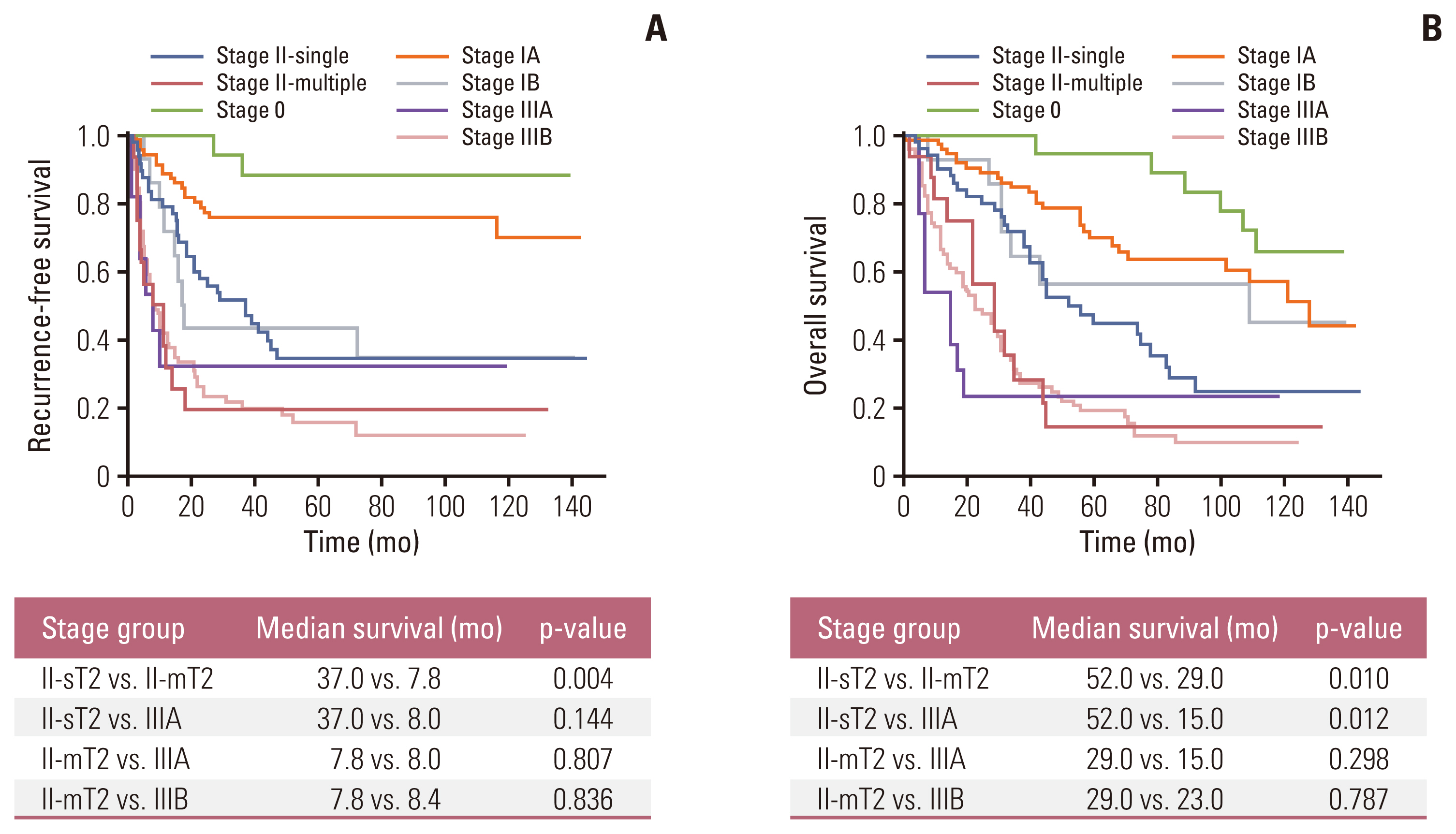
In multivariable analysis, the presence of preoperative symptoms, tumor size, lymph node ratio, multiplicity, and tumor differentiation were identified as risk factors for survival. Among 82 patients with T2, overall survival was significantly longer in patients with solitary tumors (sT2) than in those with multiple tumors (mT2) (p=0.017). Survival was compared among patients with stage II-sT2, stage II-mT2, and stage III. The stage II-sT2 group showed prolonged survival when compared with stage II-mT2 or stage III. Survivals of stage II-mT2 and stage III patients were not statistically different.
Conclusion
Tumor multiplicity was an independent risk factor for overall survival of IHCCC after surgical resection. Patients with multiple tumors showed poorer survival than patients with a single tumor. The oncologic significance of multiplicity in IHCCC should be reappraised and reflected in the next staging system update.
Figure
Reference
-
References
1. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019; 39 Suppl 1:19–31.
Article2. Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol. 2018; 7:52.
Article3. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. Cham: Springer International Publishing;2018.4. Chen Y, Weng S. Reappraisal of the T category for solitary intrahepatic cholangiocarcinoma by tumor size in 611 early-stage (T1-2N0M0) patients after hepatectomy: a Surveillance, Epidemiology, and End Results (SEER) analysis. J Gastrointest Surg. 2021; 25:1989–99.
Article5. Turner KM, Delman AM, Kharofa J, Olowokure O, Sohal D, Quillin RC, et al. A national assessment of T2 staging for intrahepatic cholangiocarcinoma and the poor prognosis associated with multifocality. Ann Surg Oncol. 2022; 29:5094–102.
Article6. Conci S, Ruzzenente A, Vigano L, Ercolani G, Fontana A, Bagante F, et al. Patterns of distribution of hepatic nodules (single, satellites or multifocal) in intrahepatic cholangiocarcinoma: prognostic impact after surgery. Ann Surg Oncol. 2018; 25:3719–27.
Article7. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer;2009.8. Lamarca A, Santos-Laso A, Utpatel K, La Casta A, Stock S, Forner A, et al. Liver metastases of intrahepatic cholangiocarcinoma: implications for an updated staging system. Hepatology. 2021; 73:2311–25.
Article9. Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, Ohgi K, et al. The evaluation of the eighth edition of the AJCC/UICC staging system for intrahepatic cholangiocarcinoma: a proposal of a modified new staging system. J Gastrointest Surg. 2020; 24:786–95.
Article10. Wright GP, Perkins S, Jones H, Zureikat AH, Marsh JW, Holtzman MP, et al. Surgical resection does not improve survival in multifocal intrahepatic cholangiocarcinoma: a comparison of surgical resection with intra-arterial therapies. Ann Surg Oncol. 2018; 25:83–90.
Article11. Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015; 17:669–80.
Article12. Li J, Moustafa M, Linecker M, Lurje G, Capobianco I, Baumgart J, et al. ALPPS for locally advanced intrahepatic cholangiocarcinoma: did aggressive surgery lead to the oncological benefit? An international multi-center study. Ann Surg Oncol. 2020; 27:1372–84.
Article13. Uenishi T, Kubo S, Yamazaki O, Yamada T, Sasaki Y, Nagano H, et al. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg. 2008; 15:417–22.14. Lamarca A, Ross P, Wasan HS, Hubner RA, McNamara MG, Lopes A, et al. Advanced intrahepatic cholangiocarcinoma: post hoc analysis of the ABC-01, -02, and -03 clinical trials. J Natl Cancer Inst. 2020; 112:200–10.
Article15. Conci S, Campagnaro T, Danese E, Lombardo E, Isa G, Vitali A, et al. Role of inflammatory and immune-nutritional prognostic markers in patients undergoing surgical resection for biliary tract cancers. Cancers (Basel). 2021; 13:3594.
Article16. Yoon SJ, Park B, Kwon J, Lim CS, Shin YC, Jung W, et al. Development of nomograms for predicting prognosis of pancreatic cancer after pancreatectomy: a multicenter study. Biomedicines. 2022; 10:1341.
Article17. Miyahara Y, Takashi S, Shimizu Y, Ohtsuka M. The prognostic impact of neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) in patients with distal bile duct cancer. World J Surg Oncol. 2020; 18:78.
Article18. Steele CW, Jamieson NB, Evans TR, McKay CJ, Sansom OJ, Morton JP, et al. Exploiting inflammation for therapeutic gain in pancreatic cancer. Br J Cancer. 2013; 108:997–1003.
Article19. Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003; 6:283–7.
Article20. Sellers CM, Uhlig J, Ludwig JM, Stein SM, Kim HS. Inflammatory markers in intrahepatic cholangiocarcinoma: effects of advanced liver disease. Cancer Med. 2019; 8:5916–29.21. Tsilimigras DI, Moris D, Mehta R, Paredes AZ, Sahara K, Guglielmi A, et al. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi-institutional analysis. HPB (Oxford). 2020; 22:1667–74.
Article22. Noguchi D, Kuriyama N, Nakagawa Y, Maeda K, Shinkai T, Gyoten K, et al. The prognostic impact of lymphocyte-to-C-reactive protein score in patients undergoing surgical resection for intrahepatic cholangiocarcinoma: a comparative study of major representative inflammatory/immunonutritional markers. PLoS One. 2021; 16:e0245946.23. Tsilimigras DI, Mehta R, Aldrighetti L, Poultsides GA, Maithel SK, Martel G, et al. Development and validation of a Laboratory Risk Score (LabScore) to predict outcomes after resection for intrahepatic cholangiocarcinoma. J Am Coll Surg. 2020; 230:381–91.
Article24. Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford). 2016; 18:79–87.
Article25. Zhang XF, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018; 105:848–56.26. Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver Int. 2019; 39(Suppl 1):143–55.27. Hu H, Xu G, Du S, Luo Z, Zhao H, Cai J. The role of lymph node dissection in intrahepatic cholangiocarcinoma: a multicenter retrospective study. BMC Surg. 2021; 21:359.
Article28. Kim SH, Han DH, Choi GH, Choi JS, Kim KS. Extent of lymph node dissection for accurate staging in intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2022; 26:70–6.
Article29. Ruzzenente A, Conci S, Vigano L, Ercolani G, Manfreda S, Bagante F, et al. Role of lymph node dissection in small (≤ 3 cm) intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2019; 23:1122–9.30. Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005; 29:728–33.
Article31. Sahara K, Tsilimigras DI, Merath K, Bagante F, Guglielmi A, Aldrighetti L, et al. Therapeutic index associated with lymphadenectomy among patients with intrahepatic cholangiocarcinoma: which patients benefit the most from nodal evaluation? Ann Surg Oncol. 2019; 26:2959–68.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histopathology of a benign bile duct lesion in the liver: Morphologic mimicker or precursor of intrahepatic cholangiocarcinoma
- Obstructive Jaundice by Tumor Emboli from Intrahepatic Cholangiocarcinoma
- Review: Analysis of Survival Rate and Prognostic Factors of Intrahepatic Cholangiocarcinoma: 318 Cases in Single Institute
- Surgical Treatment for Intrahepatic Cholangiocarcinoma
- Prognostic Factors for Intrahepatic Cholangiocarcinoma Treated with Surgical Resection