Cancer Res Treat.
2023 Jul;55(3):746-757. 10.4143/crt.2022.1541.
Immunogenicity and Safety of Vaccines against Coronavirus Disease in Actively Treated Patients with Solid Tumors: A Prospective Cohort Study
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Infectious Diseases, Department of Internal Medicine, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea
- 3Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2544158
- DOI: http://doi.org/10.4143/crt.2022.1541
Abstract
- Purpose
We aimed to assess the humoral response to and reactogenicity of coronavirus disease 2019 (COVID-19) vaccination according to the vaccine type and to analyze factors associated with immunogenicity in actively treated solid cancer patients (CPs).
Materials and Methods
Prospective cohorts of CPs, undergoing anticancer treatment, and healthcare workers (HCWs) were established. The participants had no history of previous COVID-19 and received either mRNA-based or adenovirus vector–based (AdV) vaccines as the primary series. Blood samples were collected before the first vaccination and after 2 weeks for each dose vaccination. Spike-specific binding antibodies (bAbs) in all participants and neutralizing antibodies (nAbs) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) wild-type, Delta, and Omicron variants in CPs were analyzed and presented as the geometric mean titer.
Results
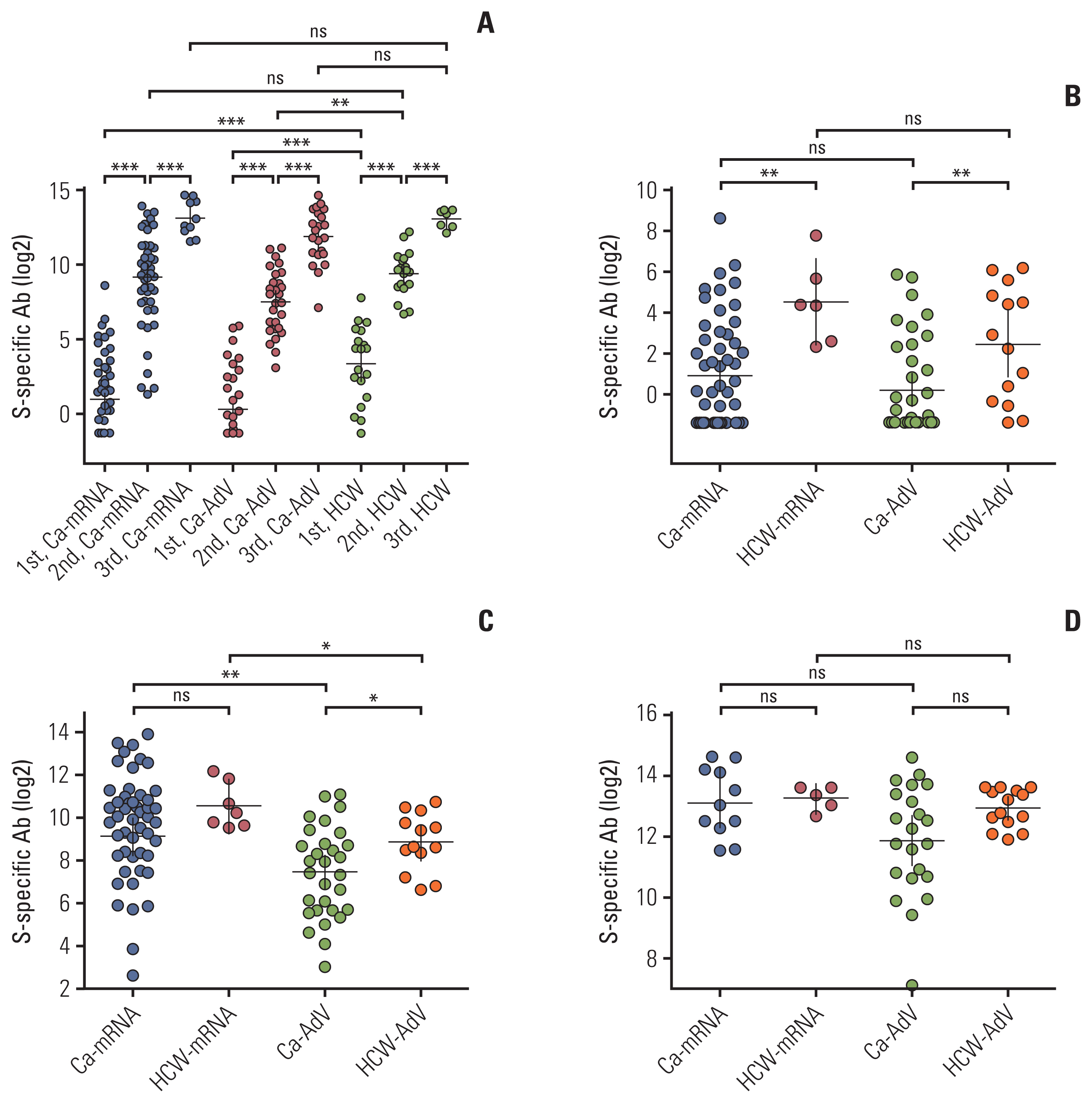
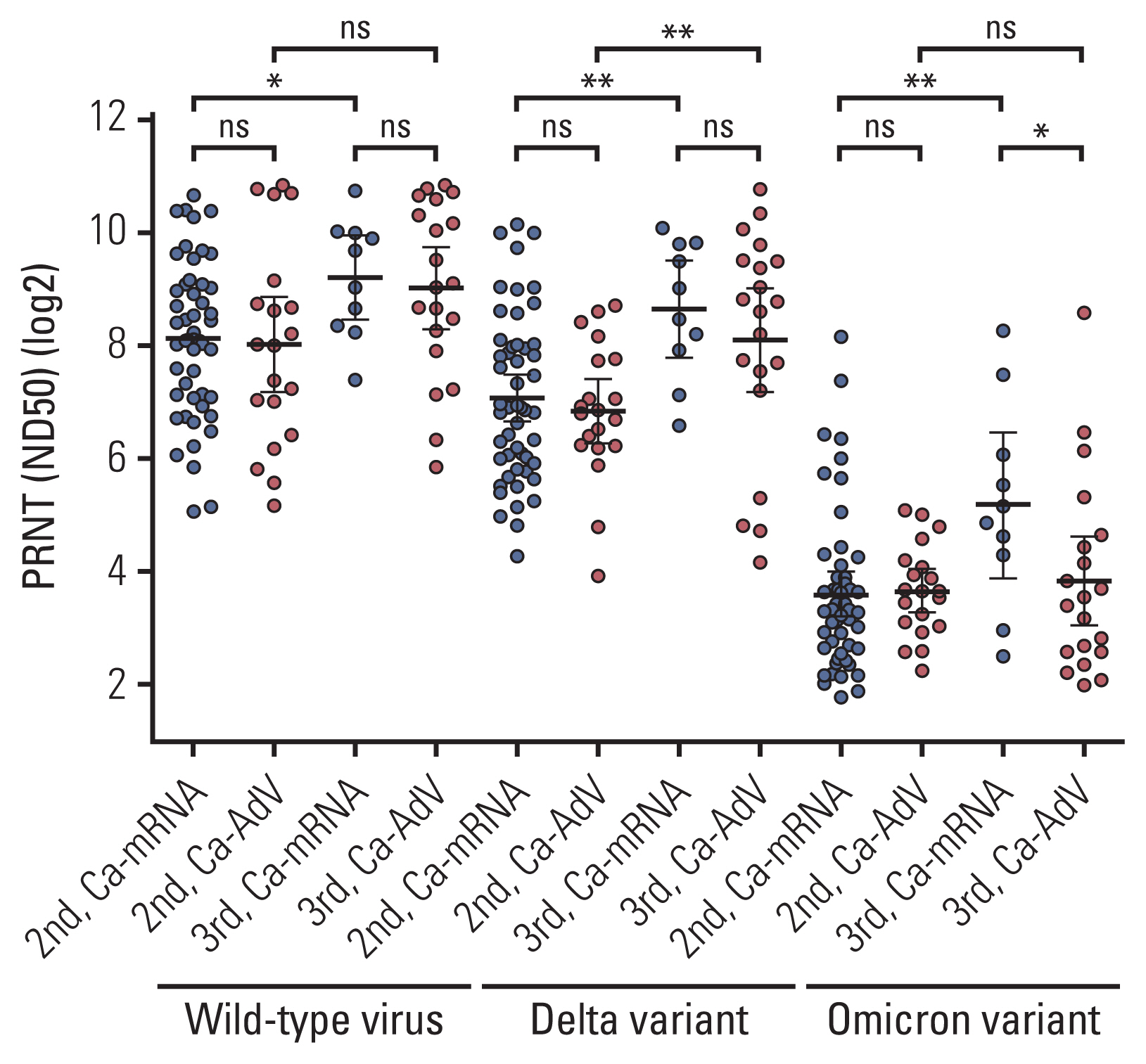
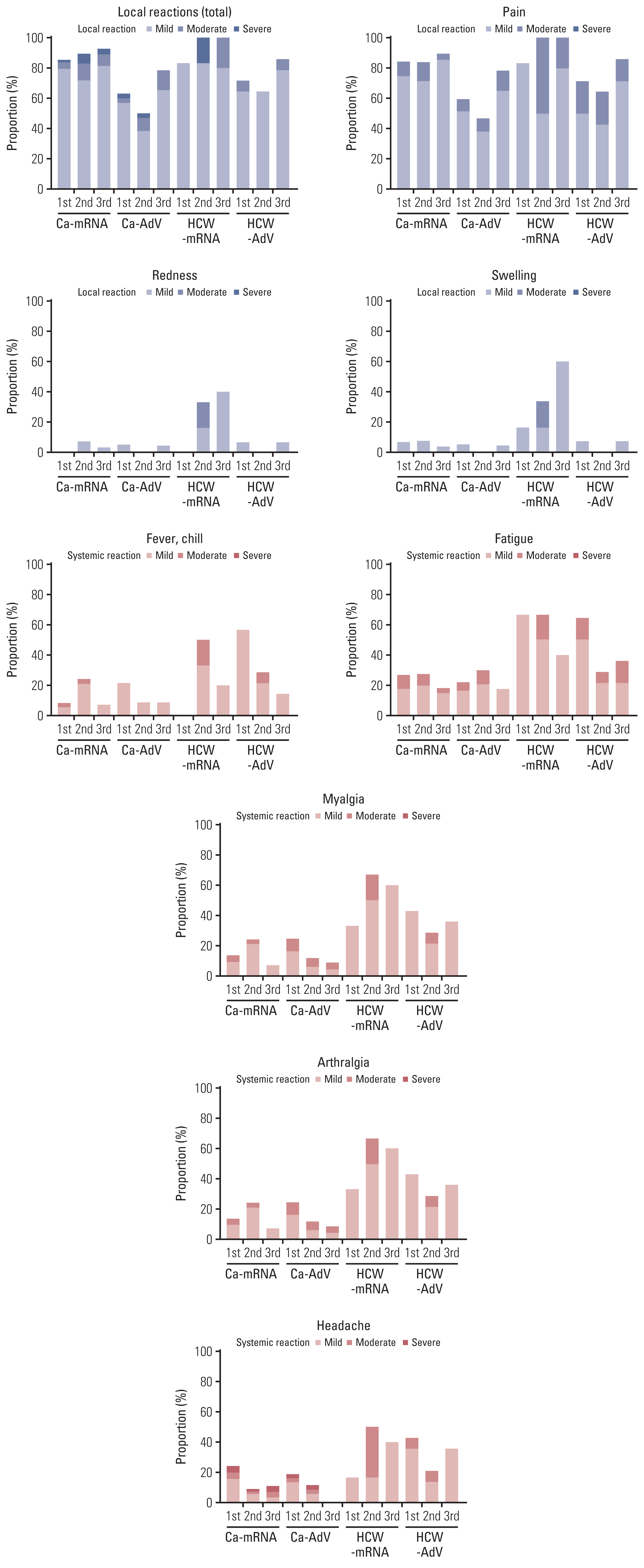
Age-matched 20 HCWs and 118 CPs were included in the analysis. The bAb seroconversion rate and antibody concentrations after the first vaccination were significantly lower in CPs than in HCWs. After the third vaccination, antibody levels in CPs with a primary series of AdV were comparable to those in HCWs, but nAb titers against the Omicron variant did not quantitatively increase in CPs with AdV vaccine as the primary series. The incidence and severity of adverse reactions post-vaccination were similar between CPs and HCWs.
Conclusion
CPs displayed delayed humoral immune response after SARS-CoV-2 vaccination. The booster dose elicited comparable bAb concentrations between CPs and HCWs, regardless of the primary vaccine type. Neutralization against the Omicron variant was not robustly elicited following the booster dose in some CPs, implying the need for additional interventions to protect them from COVID-19.
Figure
Reference
-
References
1. Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020; 43:452–5.
Article2. Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021; 7:220–7.
Article3. Tian Y, Qiu X, Wang C, Zhao J, Jiang X, Niu W, et al. Cancer associates with risk and severe events of COVID-19: a systematic review and meta-analysis. Int J Cancer. 2021; 148:363–74.
Article4. Yang L, Chai P, Yu J, Fan X. Effects of cancer on patients with COVID-19: a systematic review and meta-analysis of 63,019 participants. Cancer Biol Med. 2021; 18:298–307.
Article5. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383:2603–15.6. Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 safety and efficacy of AZD1222 (ChAd-Ox1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021; 385:2348–60.
Article7. Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, Mesa-Chavez F, Barrientos-Gutierrez T, Tagliamento M, et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022; 160:243–60.
Article8. Mishra SK, Pradhan SK, Pati S, Sahu S, Nanda RK. Waning of anti-spike antibodies in AZD1222 (ChAdOx1) vaccinated healthcare providers: a prospective longitudinal study. Cureus. 2021; 13:e19879.
Article9. Moreira ED Jr, Kitchin N, Xu X, Dychter SS, Lockhart S, Gurtman A, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022; 386:1910–21.10. Arbel R, Hammerman A, Sergienko R, Friger M, Peretz A, Netzer D, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021; 385:2413–20.
Article11. COVID-19 vaccination programme. Information for healthcare practitioners [Internet]. London: UK Health Security Agency;2022. [cited 2023 Feb 8]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1110045/COVID-19-vaccine-information-for-healthcare-practitioners-version-5.pdf.12. Preliminary public health considerations for COVID-19 vaccination strategies in the second half of 2022 [Internet]. Stockholm: European Centre for Disease Prevention and Control;2022. [cited 2023 Feb 8]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Preliminary-public-health-considerations-%20COVID-19-vaccination-2022.pdf.13. Fendler A, de Vries EGE, GeurtsvanKessel CH, Haanen JB, Wormann B, Turajlic S, et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022; 19:385–401.
Article14. Bae S, Ko JH, Choi JY, Park WJ, Lim SY, Ahn JY, et al. Heterologous ChAdOx1 and Bnt162b2 vaccination induces strong neutralizing antibody responses against SARS-CoV-2 including delta variant with tolerable reactogenicity. Clin Microbiol Infect. 2022; 28:1390.
Article15. Elecsys anti-SARS-CoV-2S instructions for use [Internet]. Silver Spring MD: U.S. Food and Drug Administration;2022. [cited 2023 Feb 8]. Available from: https://www.fda.gov/media/144037/download.16. Elecsys-Anti-SARS-CoV-2-S-factsheet-SEPT-2020, 2 [Internet]. Rotkreuz: Roche;2020. [cited 2023 Feb 8]. Available from: https://diagnostics.roche.com/content/dam/diagnostics/Blueprint/en/pdf/cps/Elecsys-Anti-SARS-CoV-2-S-factsheet-SEPT-2020-2.pdf.17. Center for Biologics Evaluation and Research. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Rockville, MD: U.S. Food and Drug Administration;2009.18. Fendler A, Shepherd ST, Au L, Wilkinson KA, Wu M, Byrne F, et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer. 2021; 2:1305–20.19. Naranbhai V, Pernat CA, Gavralidis A, St Denis KJ, Lam EC, Spring LM, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J Clin Oncol. 2022; 40:12–23.
Article20. Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez-Lugo JD, et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2022; 40:3–5.
Article21. Lasagna A, Bergami F, Lilleri D, Percivalle E, Quaccini M, Alessio N, et al. Immunogenicity and safety after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with solid tumors on active treatment: a prospective cohort study. ESMO Open. 2022; 7:100458.
Article22. Naranbhai V, St Denis KJ, Lam EC, Ofoman O, Garcia-Beltran WF, Mairena CB, et al. Neutralization breadth of SARS-CoV-2 viral variants following primary series and booster SARS-CoV-2 vaccines in patients with cancer. Cancer Cell. 2022; 40:103–8.
Article23. Rossler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022; 386:698–700.
Article24. Agbarya A, Sarel I, Ziv-Baran T, Agranat S, Schwartz O, Shai A, et al. Efficacy of the mRNA-based BNT162b2 COVID-19 vaccine in patients with solid malignancies treated with anti-neoplastic drugs. Cancers (Basel). 2021; 13:4191.
Article25. Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021; 39:1081–90.
Article26. Ligumsky H, Safadi E, Etan T, Vaknin N, Waller M, Croll A, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst. 2022; 114:203–9.
Article27. Nelli F, Fabbri A, Onorato A, Giannarelli D, Silvestri MA, Giron Berrios JR, et al. Effects of active cancer treatment on safety and immunogenicity of COVID-19 mRNA-BNT162b2 vaccine: preliminary results from the prospective observational Vax-On study. Ann Oncol. 2022; 33:107–8.
Article28. Brest P, Mograbi B, Hofman P, Milano G. COVID-19 vaccination and cancer immunotherapy: should they stick together? Br J Cancer. 2022; 126:1–3.29. Piening A, Ebert E, Khojandi N, Alspach E, Teague RM. Immune responses to SARS-CoV-2 in vaccinated patients receiving checkpoint blockade immunotherapy for cancer. Front Immunol. 2022; 13:1022732.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Updates on the coronavirus disease 2019 vaccine and consideration in children
- Sleep and vaccine administration time as factors influencing vaccine immunogenicity
- Newcastle disease virus vectored vaccines as bivalent or antigen delivery vaccines
- Vaccines development in India: advances, regulation, and challenges
- The Immunogenicity and Safety of the Live-attenuated SA 14-14-2 Japanese Encephalitis Vaccine Given with a Two-dose Primary Schedule in Children