Anat Cell Biol.
2023 Jun;56(2):166-178. 10.5115/acb.22.190.
Tree of life: endothelial cell in norm and disease, the good guy is a partner in crime!
- Affiliations
-
- 1National Research Mordovia State University, Saransk, Mordovia
- KMID: 2544077
- DOI: http://doi.org/10.5115/acb.22.190
Abstract
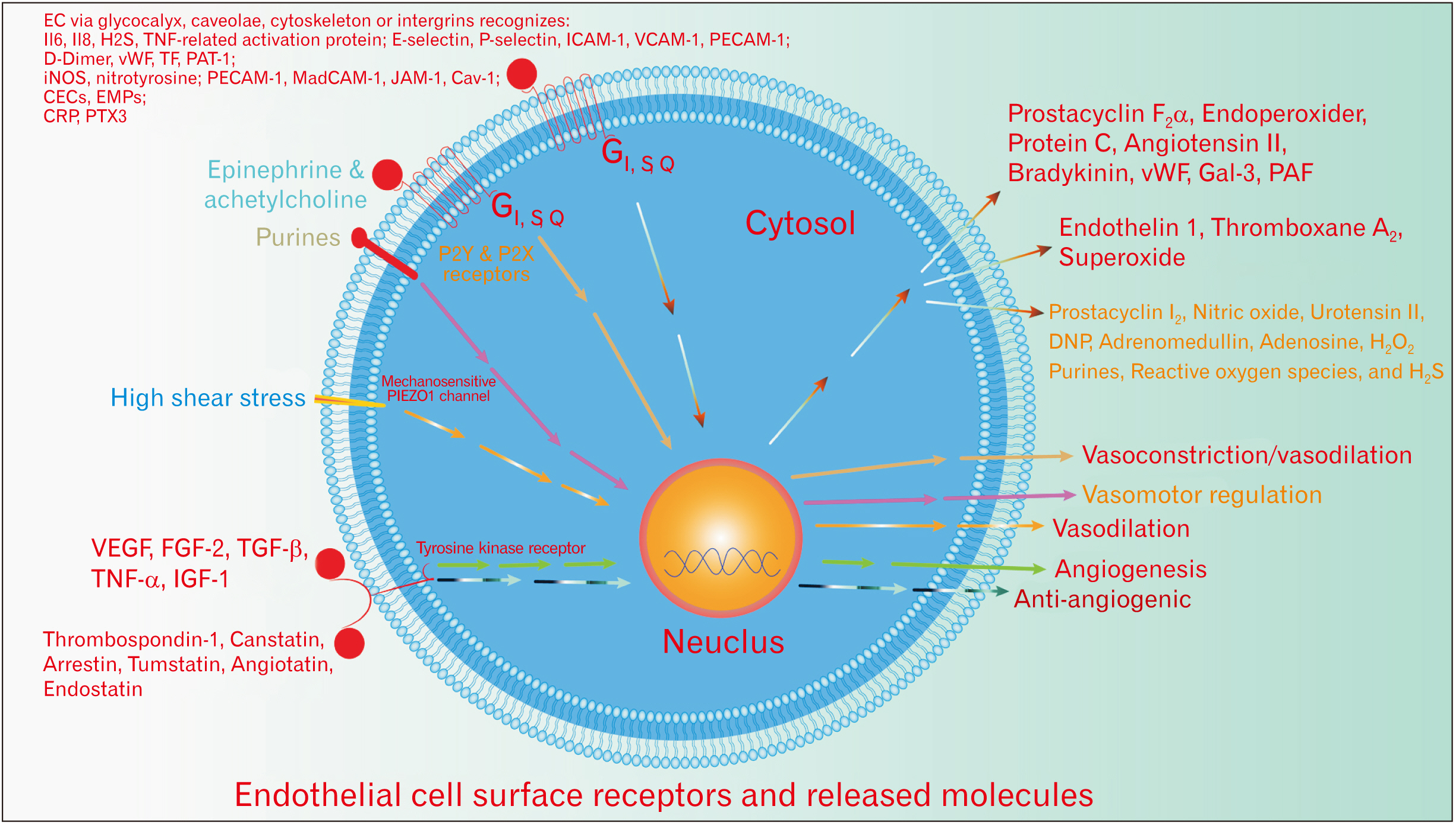
- Undeniably, endothelial cells (EC) contribute to the maintenance of the homeostasis of the organism through modulating cellular physiology, including signaling pathways, through the release of highly active molecules as well as the response to a myriad of extrinsic and intrinsic signaling factors. Review the data from the current literature on the EC role in norm and disease. Endothelium maintains a precise balance between the released molecules, where EC dysfunction arises when the endothelium actions shift toward vasoconstriction, the proinflammatory, prothrombic properties after the alteration of nitric oxide (NO) production and oxidative stress. The functions of the EC are regulated by the negative/ positive feedback from the organism, through EC surface receptors, and the crosstalk between NO, adrenergic receptors, and oxidative stress. More than a hundred substances can interact with EC. The EC dysfunction is a hallmark in the emergence and progression of vascular-related pathologies. The paper concisely reviews recent advances in EC (patho) physiology. Grasping EC physiology is crucial to gauge their potential clinical utility and optimize the current therapies as well as to establish novel nanotherapeutic molecular targets include; endothelial receptors, cell adhesion molecules, integrins, signaling pathways, enzymes; peptidases.
Keyword
Figure
Reference
-
References
1. Bierhansl L, Conradi LC, Treps L, Dewerchin M, Carmeliet P. 2017; Central role of metabolism in endothelial cell function and vascular disease. Physiology (Bethesda). 32:126–40. DOI: 10.1152/physiol.00031.2016. PMID: 28202623. PMCID: PMC5337830.
Article2. Khan S, Taverna F, Rohlenova K, Treps L, Geldhof V, de Rooij L, Sokol L, Pircher A, Conradi LC, Kalucka J, Schoonjans L, Eelen G, Dewerchin M, Karakach T, Li X, Goveia J, Carmeliet P. 2019; EndoDB: a database of endothelial cell transcriptomics data. Nucleic Acids Res. 47(D1):D736–44. DOI: 10.1093/nar/gky997. PMID: 30357379. PMCID: PMC6324065.
Article3. Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. 2013; The vascular endothelium and human diseases. Int J Biol Sci. 9:1057–69. DOI: 10.7150/ijbs.7502. PMID: 24250251. PMCID: PMC3831119.
Article4. Baselet B, Sonveaux P, Baatout S, Aerts A. 2019; Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell Mol Life Sci. 76:699–728. DOI: 10.1007/s00018-018-2956-z. PMID: 30377700. PMCID: PMC6514067.
Article5. Zhang J, Tecson KM, McCullough PA. 2021; Role of endothelial cell receptors in the context of SARS-CoV-2 infection (COVID-19). Proc (Bayl Univ Med Cent). 34:262–8. DOI: 10.1080/08998280.2021.1874231. PMID: 33664552. PMCID: PMC7852287.
Article6. Félétou M. 2011; The endothelium: part 1: multiple functions of the endothelial cells-focus on endothelium-derived vasoactive mediators. Colloq Ser Integr Syst Physiol. 3:1–306. DOI: 10.4199/C00031ED1V01Y201105ISP019. PMID: 21850763.
Article7. Tian D, Teng X, Jin S, Chen Y, Xue H, Xiao L, Wu Y. 2020; Endogenous hydrogen sulfide improves vascular remodeling through PPARδ/SOCS3 signaling. J Adv Res. 27:115–25. DOI: 10.1016/j.jare.2020.06.005. PMID: 33318871. PMCID: PMC7728593.
Article8. Shuvaev VV, Brenner JS, Muzykantov VR. 2015; Targeted endothelial nanomedicine for common acute pathological conditions. J Control Release. 219:576–95. DOI: 10.1016/j.jconrel.2015.09.055. PMID: 26435455. PMCID: PMC5450650.
Article9. Jambusaria A, Hong Z, Zhang L, ivastava S Sr, Jana A, Toth PT, Dai Y, Malik AB, Rehman J. 2020; Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. Elife. 9:e51413. DOI: 10.7554/eLife.51413. PMID: 31944177. PMCID: PMC7002042. PMID: 48432eae5df34dc8902eb012a4ba6d9b.
Article10. Davies PF. 2009; Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 6:16–26. DOI: 10.1038/ncpcardio1397. PMID: 19029993. PMCID: PMC2851404.
Article11. Marzoog BA, Vlasova TI. 2022; Apr. 29. The metabolic syndrome puzzles; possible pathogenesis and management. Curr Diabetes Rev. [Epub]. https://doi.org/10.2174/1573399818666220429100411. DOI: 10.2174/1573399818666220429100411. PMID: 35507784.
Article12. Marzoog BA. 2022; Recent advances in molecular biology of metabolic syndrome pathophysiology: endothelial dysfunction as a potential therapeutic target. J Diabetes Metab Disord. 21:1903–11. DOI: 10.1007/s40200-022-01088-y. PMID: 36065330. PMCID: PMC9430013.
Article13. Marzoog B. 2022; Lipid behavior in metabolic syndrome pathophysiology. Curr Diabetes Rev. 18:e150921196497. DOI: 10.2174/1573399817666210915101321. PMID: 34525924.
Article14. Thakore P, Earley S. 2019; Transient receptor potential channels and endothelial cell calcium signaling. Compr Physiol. 9:1249–77. DOI: 10.1002/cphy.c180034. PMID: 31187891. PMCID: PMC6724529.
Article15. Dalal PJ, Muller WA, Sullivan DP. 2020; Endothelial cell calcium signaling during barrier function and inflammation. Am J Pathol. 190:535–42. DOI: 10.1016/j.ajpath.2019.11.004. PMID: 31866349. PMCID: PMC7074364.
Article16. Evans CE, Iruela-Arispe ML, Zhao YY. 2021; Mechanisms of endothelial regeneration and vascular repair and their application to regenerative medicine. Am J Pathol. 191:52–65. DOI: 10.1016/j.ajpath.2020.10.001. PMID: 33069720. PMCID: PMC7560161.
Article17. Hu Y, Chen M, Wang M, Li X. 2022; Flow-mediated vasodilation through mechanosensitive G protein-coupled receptors in endothelial cells. Trends Cardiovasc Med. 32:61–70. DOI: 10.1016/j.tcm.2020.12.010. PMID: 33406458.
Article18. Pernomian L, do Prado AF, Silva BR, de Paula TD, Grando MD, Bendhack LM. 2021; C-type natriuretic peptide-induced relaxation through cGMP-dependent protein kinase and SERCA activation is impaired in two kidney-one clip rat aorta. Life Sci. 272:119223. DOI: 10.1016/j.lfs.2021.119223. PMID: 33610574.
Article19. Wettschureck N, Strilic B, Offermanns S. 2019; Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol Rev. 99:1467–525. DOI: 10.1152/physrev.00037.2018. PMID: 31140373.
Article20. Motawe ZY, Abdelmaboud SS, Breslin JW. 2021; Involvement of sigma receptor-1 in lymphatic endothelial barrier integrity and bioenergetic regulation. Lymphat Res Biol. 19:231–9. DOI: 10.1089/lrb.2020.0060. PMID: 33226886. PMCID: PMC8220569.
Article21. Frees A, Assersen KB, Jensen M, Hansen PBL, Vanhoutte PM, Madsen K, Federlein A, Lund L, Toft A, Jensen BL. 2021; Natriuretic peptides relax human intrarenal arteries through natriuretic peptide receptor type-A recapitulated by soluble guanylyl cyclase agonists. Acta Physiol (Oxf). 231:e13565. DOI: 10.1111/apha.13565. PMID: 33010104.
Article22. Tao BB, Liu SY, Zhang CC, Fu W, Cai WJ, Wang Y, Shen Q, Wang MJ, Chen Y, Zhang LJ, Zhu YZ, Zhu YC. 2013; VEGFR2 functions as an H2S-targeting receptor protein kinase with its novel Cys1045-Cys1024 disulfide bond serving as a specific molecular switch for hydrogen sulfide actions in vascular endothelial cells. Antioxid Redox Signal. 19:448–64. DOI: 10.1089/ars.2012.4565. PMID: 23199280. PMCID: PMC3704125.23. Xiao L, Dong JH, Teng X, Jin S, Xue HM, Liu SY, Guo Q, Shen W, Ni XC, Wu YM. 2018; Hydrogen sulfide improves endothelial dysfunction in hypertension by activating peroxisome proliferator-activated receptor delta/endothelial nitric oxide synthase signaling. J Hypertens. 36:651–65. DOI: 10.1097/HJH.0000000000001605. PMID: 29084084.
Article24. Zuccolo E, Laforenza U, Negri S, Botta L, Berra-Romani R, Faris P, Scarpellino G, Forcaia G, Pellavio G, Sancini G, Moccia F. 2019; Muscarinic M5 receptors trigger acetylcholine-induced Ca2+ signals and nitric oxide release in human brain microvascular endothelial cells. J Cell Physiol. 234:4540–62. DOI: 10.1002/jcp.27234. PMID: 30191989.
Article25. Berra-Romani R, Faris P, Pellavio G, Orgiu M, Negri S, Forcaia G, Var-Gaz-Guadarrama V, Garcia-Carrasco M, Botta L, Sancini G, Laforenza U, Moccia F. 2020; Histamine induces intracellular Ca2+ oscillations and nitric oxide release in endothelial cells from brain microvascular circulation. J Cell Physiol. 235:1515–30. DOI: 10.1002/jcp.29071. PMID: 31310018.26. Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. 2018; DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46(D1):D1074–82. DOI: 10.1093/nar/gkx1037. PMID: 29126136. PMCID: PMC5753335.
Article27. Tao BB, Cai WJ, Zhu YC. 2015; H2S is a promoter of angiogenesis: identification of H2S "receptors" and its molecular switches in vascular endothelial cells. Handb Exp Pharmacol. 230:137–52. DOI: 10.1007/978-3-319-18144-8_6. PMID: 26162832.
Article28. Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. 2016; Endothelin. Pharmacol Rev. 68:357–418. DOI: 10.1124/pr.115.011833. PMID: 26956245. PMCID: PMC4815360.
Article29. Maguire JJ, Davenport AP. 2015; Endothelin receptors and their antagonists. Semin Nephrol. 35:125–36. DOI: 10.1016/j.semnephrol.2015.02.002. PMID: 25966344. PMCID: PMC4437774.
Article30. Kowalczyk A, Kleniewska P, Kolodziejczyk M, Skibska B, Goraca A. 2015; The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz). 63:41–52. DOI: 10.1007/s00005-014-0310-1. PMID: 25288367. PMCID: PMC4289534.
Article31. Abdullah Marzoog B. 2023; Adaptive and compensatory mechanisms of the cardiovascular system and disease risk factors in young males and females. New Emir Med J. 4:e281122211293. DOI: 10.2174/04666221128110145.
Article32. Marzoog BA. 2022; Oct. 21. Endothelial cell autophagy in the context of disease development. Anat Cell Biol. [Epub]. https://doi.org/10.5115/acb.22.098. DOI: 10.5115/acb.22.098. PMID: 36267005. PMCID: PMC9989784.
Article33. Motegi S. Takehara K, Fujimoto M, Kuwana M, editors. 2016. Endothelin. Systemic Sclerosis. Springer;p. 155–71. DOI: 10.1007/978-4-431-55708-1_10.
Article34. Schiffrin EL. 2018; Does endothelin-1 raise or lower blood pressure in humans? Nephron. 139:47–50. DOI: 10.1159/000487346. PMID: 29448245.
Article35. Houde M, Desbiens L, D'Orléans-Juste P. 2016; Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol. 77:143–75. DOI: 10.1016/bs.apha.2016.05.002. PMID: 27451097.36. Sousa J, Diniz C. Lenasi H, editor. 2018. Vascular sympathetic neurotransmission and endothelial dysfunction. Endothelial Dysfunction. IntechOpen;DOI: 10.5772/intechopen.72442.
Article37. Conti V, Russomanno G, Corbi G, Izzo V, Vecchione C, Filippelli A. 2013; Adrenoreceptors and nitric oxide in the cardiovascular system. Front Physiol. 4:321. DOI: 10.3389/fphys.2013.00321. PMID: 24223559. PMCID: PMC3818479. PMID: 736af3961f2b43fe85c5a52e17988f17.
Article38. Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. 2017; Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol (Oxf). 219:22–96. DOI: 10.1111/apha.12646. PMID: 26706498.
Article39. Iring A, Jin YJ, Albarrán-Juárez J, Siragusa M, Wang S, Dancs PT, Nakayama A, Tonack S, Chen M, Künne C, Sokol AM, Günther S, Martínez A, Fleming I, Wettschureck N, Graumann J, Weinstein LS, Offermanns S. 2019; Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J Clin Invest. 129:2775–91. DOI: 10.1172/JCI123825. PMID: 31205027. PMCID: PMC6597232.
Article40. Voors AA, Kremer D, Geven C, Ter Maaten JM, Struck J, Bergmann A, Pickkers P, Metra M, Mebazaa A, Düngen HD, Butler J. 2019; Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur J Heart Fail. 21:163–71. DOI: 10.1002/ejhf.1366. PMID: 30592365. PMCID: PMC6607488.
Article41. Li X, Sun X, Carmeliet P. 2019; Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 30:414–33. DOI: 10.1016/j.cmet.2019.08.011. PMID: 31484054.
Article42. Vestweber D. 2021; Vascular endothelial protein tyrosine phosphatase regulates endothelial function. Physiology (Bethesda). 36:84–93. DOI: 10.1152/physiol.00026.2020. PMID: 33595386.
Article43. Randi AM, Smith KE, Castaman G. 2018; von Willebrand factor regulation of blood vessel formation. Blood. 132:132–40. DOI: 10.1182/blood-2018-01-769018. PMID: 29866817. PMCID: PMC6182264.
Article44. Nguyen TS, Lapidot T, Ruf W. 2018; Extravascular coagulation in hematopoietic stem and progenitor cell regulation. Blood. 132:123–31. DOI: 10.1182/blood-2017-12-768986. PMID: 29866813. PMCID: PMC6634957.
Article45. Randi AM, Laffan MA. 2017; Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost. 15:13–20. DOI: 10.1111/jth.13551. PMID: 27778439.
Article46. Daly C, Eichten A, Castanaro C, Pasnikowski E, Adler A, Lalani AS, Papadopoulos N, Kyle AH, Minchinton AI, Yancopoulos GD, Thurston G. 2013; Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res. 73:108–18. DOI: 10.1158/0008-5472.CAN-12-2064. PMID: 23149917.
Article47. Funasaka T, Raz A, Nangia-Makker P. 2014; Galectin-3 in angiogenesis and metastasis. Glycobiology. 24:886–91. DOI: 10.1093/glycob/cwu086. PMID: 25138305. PMCID: PMC4153760.
Article48. Kim SJ, Chun KH. 2020; Non-classical role of Galectin-3 in cancer progression: translocation to nucleus by carbohydrate-recognition independent manner. BMB Rep. 53:173–80. DOI: 10.5483/BMBRep.2020.53.4.020. PMID: 32172730. PMCID: PMC7196190.
Article49. Groeneveld DJ, Sanders YV, Adelmeijer J, Mauser-Bunschoten EP, van der Bom JG, Cnossen MH, Fijnvandraat K, Laros-van Gorkom BAP, Meijer K, Lisman T, Eikenboom J, Leebeek FWG. 2018; Circulating angiogenic mediators in patients with moderate and severe von Willebrand disease: a multicentre cross-sectional study. Thromb Haemost. 118:152–60. DOI: 10.1160/TH17-06-0397. PMID: 29304535.
Article50. Hepner M, Karlaftis V. 2013; Protein C. Methods Mol Biol. 992:365–72. DOI: 10.1007/978-1-62703-339-8_29. PMID: 23546729.
Article51. Giri H, Cai X, Panicker SR, Biswas I, Rezaie AR. 2019; Thrombomodulin regulation of mitogen-activated protein kinases. Int J Mol Sci. 20:1851. DOI: 10.3390/ijms20081851. PMID: 30991642. PMCID: PMC6514922. PMID: 239b044c6fa24302aea9e478ae983c81.
Article52. Hepner M, Karlaftis V. 2013; Protein S. Methods Mol Biol. 992:373–81. DOI: 10.1007/978-1-62703-339-8_30. PMID: 23546730.
Article53. Griffin JH, Zlokovic BV, Mosnier LO. 2018; Activated protein C, protease activated receptor 1, and neuroprotection. Blood. 132:159–69. DOI: 10.1182/blood-2018-02-769026. PMID: 29866816. PMCID: PMC6043978.
Article54. Julovi SM, Shen K, Mckelvey K, Minhas N, March L, Jackson CJ. 2013; Activated protein C inhibits proliferation and tumor necrosis factor α-stimulated activation of p38, c-Jun NH2-terminal kinase (JNK) and Akt in rheumatoid synovial fibroblasts. Mol Med. 19:324–31. DOI: 10.2119/molmed.2013.00034. PMID: 24096826. PMCID: PMC4344457.
Article55. Healy LD, Puy C, Fernández JA, Mitrugno A, Keshari RS, Taku NA, Chu TT, Xu X, Gruber A, Lupu F, Griffin JH, McCarty OJT. 2017; Activated protein C inhibits neutrophil extracellular trap formation in vitro and activation in vivo. J Biol Chem. 292:8616–29. DOI: 10.1074/jbc.M116.768309. PMID: 28408624. PMCID: PMC5448091.56. Loghmani H, Conway EM. 2018; Exploring traditional and nontraditional roles for thrombomodulin. Blood. 132:148–58. DOI: 10.1182/blood-2017-12-768994. PMID: 29866818.
Article57. Ito T, Kakihana Y, Maruyama I. 2016; Thrombomodulin as an intravascular safeguard against inflammatory and thrombotic diseases. Expert Opin Ther Targets. 20:151–8. DOI: 10.1517/14728222.2016.1086750. PMID: 26558419.
Article58. Pan B, Wang X, Nishioka C, Honda G, Yokoyama A, Zeng L, Xu K, Ikezoe T. 2017; G-protein coupled receptor 15 mediates angiogenesis and cytoprotective function of thrombomodulin. Sci Rep. 7:692. DOI: 10.1038/s41598-017-00781-w. PMID: 28386128. PMCID: PMC5429650. PMID: cd8c9b4f5e79444090f11c54054dee9b.
Article59. Son BK, Akishita M, Iijima K, Ogawa S, Arai T, Ishii H, Maemura K, Aburatani H, Eto M, Ouchi Y. 2013; Thrombomodulin, a novel molecule regulating inorganic phosphate-induced vascular smooth muscle cell calcification. J Mol Cell Cardiol. 56:72–80. DOI: 10.1016/j.yjmcc.2012.12.013. PMID: 23274063.
Article60. Chen J, Chung DW. 2018; Inflammation, von Willebrand factor, and ADAMTS13. Blood. 132:141–7. DOI: 10.1182/blood-2018-02-769000. PMID: 29866815. PMCID: PMC6043979.
Article61. Marzoog BA, Vlasova TI. 2021; Membrane lipids under norm and pathology. Eur J Clin Exp Med. 19:59–75. DOI: 10.15584/ejcem.2021.1.9.
Article62. Heldin CH, Moustakas A. 2016; Signaling receptors for TGF-β family members. Cold Spring Harb Perspect Biol. 8:a022053. DOI: 10.1101/cshperspect.a022053. PMID: 27481709. PMCID: PMC4968163.
Article63. Xie X, Sun W, Wang J, Li X, Liu X, Liu N. 2017; Activation of thromboxane A2 receptors mediates endothelial dysfunction in diabetic mice. Clin Exp Hypertens. 39:312–8. DOI: 10.1080/10641963.2016.1246558. PMID: 28513223.
Article64. Ellinsworth DC, Shukla N, Fleming I, Jeremy JY. 2014; Interactions between thromboxane A₂, thromboxane/prostaglandin (TP) receptors, and endothelium-derived hyperpolarization. Cardiovasc Res. 102:9–16. DOI: 10.1093/cvr/cvu015. PMID: 24469536.
Article65. Ding J, Yu M, Jiang J, Luo Y, Zhang Q, Wang S, Yang F, Wang A, Wang L, Zhuang M, Wu S, Zhang Q, Xia Y, Lu D. 2020; Angiotensin II decreases endothelial nitric oxide synthase phosphorylation via AT1R Nox/ROS/PP2A pathway. Front Physiol. 11:566410. DOI: 10.3389/fphys.2020.566410. PMID: 33162896. PMCID: PMC7580705. PMID: d850ebab98074c969807b3972cc481bf.
Article66. Walker M, Green J, Ferrie R, Cook-Mills J. 2017; Serotonin receptor regulation of eosinophil transendothelial migration. FASEB J. 31:55.67. Saternos HC, Almarghalani DA, Gibson HM, Meqdad MA, Antypas RB, Lingireddy A, AbouAlaiwi WA. 2018; Distribution and function of the muscarinic receptor subtypes in the cardiovascular system. Physiol Genomics. 50:1–9. DOI: 10.1152/physiolgenomics.00062.2017. PMID: 29093194.68. Radu BM, Osculati AMM, Suku E, Banciu A, Tsenov G, Merigo F, Di Chio M, Banciu DD, Tognoli C, Kacer P, Giorgetti A, Radu M, Bertini G, Fabene PF. 2017; All muscarinic acetylcholine receptors (M1-M5) are expressed in murine brain microvascular endothelium. Sci Rep. 7:5083. DOI: 10.1038/s41598-017-05384-z. PMID: 28698560. PMCID: PMC5506046. PMID: 02226d4ad5a34ae38427c894c235be2a.69. Wilson C, Lee MD, McCarron JG. 2016; Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J Physiol. 594:7267–307. DOI: 10.1113/JP272927. PMID: 27730645. PMCID: PMC5157078.
Article70. Yu L, Dai Y, Mineo C. 2021; Novel functions of endothelial scavenger receptor class B Type I. Curr Atheroscler Rep. 23:6. DOI: 10.1007/s11883-020-00903-2. PMID: 33420646. PMCID: PMC8918055.71. Kashefiolasl S, Leisegang MS, Helfinger V, Schürmann C, Pflüger-Müller B, Randriamboavonjy V, Vasconez AE, Carmeliet G, Badenhoop K, Hintereder G, Seifert V, Schröder K, Konczalla J, Brandes RP. 2021; Vitamin D-A new perspective in treatment of cerebral vasospasm. Neurosurgery. 88:674–85. DOI: 10.1093/neuros/nyaa484. PMID: 33269399. PMCID: PMC7884149.
Article72. Chaudhuri P, Rosenbaum MA, Sinharoy P, Damron DS, Birnbaumer L, Graham LM. 2016; Membrane translocation of TRPC6 channels and endothelial migration are regulated by calmodulin and PI3 kinase activation. Proc Natl Acad Sci U S A. 113:2110–5. DOI: 10.1073/pnas.1600371113. PMID: 26858457. PMCID: PMC4776520.
Article73. Dalal PJ, Sullivan DP, Muller WA. 2019; Endothelial calmodulin and CaMKII play a role in leukocyte transmigration. FASEB J. 33:375. DOI: 10.1096/fasebj.2019.33.1_supplement.375.5.
Article74. Shihoya W, Nishizawa T, Okuta A, Tani K, Dohmae N, Fujiyoshi Y, Nureki O, Doi T. 2016; Activation mechanism of endothelin ETB receptor by endothelin-1. Nature. 537:363–8. DOI: 10.1038/nature19319. PMID: 27595334.
Article75. Xu S, Wen H, Jiang H. 2012; Urotensin II promotes the proliferation of endothelial progenitor cells through p38 and p44/42 MAPK activation. Mol Med Rep. 6:197–200. DOI: 10.3892/mmr.2012.899. PMID: 22552405.76. Ashraf MA, Nookala V. 2022. Biochemistry of platelet activating factor. StatPearls. StatPearls Publishing;Treasure Island: DOI: 10.1007/springerreference_39453.77. Ralevic V, Dunn WR. 2015; Purinergic transmission in blood vessels. Auton Neurosci. 191:48–66. DOI: 10.1016/j.autneu.2015.04.007. PMID: 26004513.78. Berendam SJ, Koeppel AF, Godfrey NR, Rouhani SJ, Woods AN, Rodriguez AB, Peske JD, Cummings KL, Turner SD, Engelhard VH. 2019; Comparative transcriptomic analysis identifies a range of immunologically related functional elaborations of lymph node associated lymphatic and blood endothelial cells. Front Immunol. 10:816. DOI: 10.3389/fimmu.2019.00816. PMID: 31057546. PMCID: PMC6478037. PMID: db1eca5d9c874b308d3564b91c460711.
Article79. Carman CV, Martinelli R. Bradshaw RA, Stahl PD, editors. 2016. Lymphocyte-endothelial interactions. Encyclopedia of Cell Biology. Elsevier;p. 632–49. DOI: 10.1016/B978-0-12-394447-4.30095-5.
Article80. Ribatti D. Ribatti D, editor. 2017. The origins of lymphatic vessels: an historical review. Milestones in Immunology. Elsevier;p. 129–62. DOI: 10.1016/B978-0-12-811313-4.00010-3.81. Keuschnigg J, Karinen S, Auvinen K, Irjala H, Mpindi JP, Kallioniemi O, Hautaniemi S, Jalkanen S, Salmi M. 2013; Plasticity of blood- and lymphatic endothelial cells and marker identification. PLoS One. 8:e74293. DOI: 10.1371/journal.pone.0074293. PMID: 24058540. PMCID: PMC3769239. PMID: aa5f6f4cdead469a84ca43ce114f6dd0.
Article82. Yang J, Zhang S, Zhang L, Xie X, Wang H, Jie Z, Gu M, Yang JY, Cheng X, Sun SC. 2019; Lymphatic endothelial cells regulate B-cell homing to lymph nodes via a NIK-dependent mechanism. Cell Mol Immunol. 16:165–77. DOI: 10.1038/cmi.2017.167. PMID: 29503445. PMCID: PMC6355805.
Article83. Russo E, Runge P, Jahromi NH, Naboth H, Landtwing A, Montecchi R, Leicht N, Hunter MC, Takai Y, Halin C. 2021; CD112 regulates angiogenesis and T cell entry into the spleen. Cells. 10:169. DOI: 10.3390/cells10010169. PMID: 33467729. PMCID: PMC7830896. PMID: 48c9bb8f0a614e5d888c34399f17462b.84. Claro V, Ferro A. 2020; Netrin-1: focus on its role in cardiovascular physiology and atherosclerosis. JRSM Cardiovasc Dis. 9:2048004020959574. DOI: 10.1177/2048004020959574. PMID: 33282228. PMCID: PMC7691900. PMID: 6d1ae565a2334a41a52233a84b211e6e.
Article85. Dieterich LC, Tacconi C, Menzi F, Proulx ST, Kapaklikaya K, Hamada M, Takahashi S, Detmar M. 2020; Lymphatic MAFB regulates vascular patterning during developmental and pathological lymphangiogenesis. Angiogenesis. 23:411–23. DOI: 10.1007/s10456-020-09721-1. PMID: 32307629. PMCID: PMC7311381.
Article86. Jourde-Chiche N, Fakhouri F, Dou L, Bellien J, Burtey S, Frimat M, Jarrot PA, Kaplanski G, Le Quintrec M, Pernin V, Rigothier C, Sallée M, Fremeaux-Bacchi V, Guerrot D, Roumenina LT. 2019; Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 15:87–108. DOI: 10.1038/s41581-018-0098-z. PMID: 30607032.87. Becker PW, Sacilotto N, Nornes S, Neal A, Thomas MO, Liu K, Preece C, Ratnayaka I, Davies B, Bou-Gharios G, De Val S. 2016; An intronic Flk1 enhancer directs arterial-specific expression via RBPJ-mediated venous repression. Arterioscler Thromb Vasc Biol. 36:1209–19. DOI: 10.1161/ATVBAHA.116.307517. PMID: 27079877. PMCID: PMC4894770.88. Marzoog BA. 2022; Systemic and local hypothermia in the context of cell regeneration. Cryo Letters. 43:66–73. DOI: 10.54680/fr22210110112. PMID: 36626147.
Article89. Marzoog BA, Vlasova TI. 2022; Myocardiocyte autophagy in the context of myocardiocytes regeneration: a potential novel therapeutic strategy. Egypt J Med Hum Genet. 23:41. DOI: 10.1186/s43042-022-00250-8. PMID: d1da3ab8dd21477095572d54dc7e6bc3.
Article90. Bubb KJ, Aubdool AA, Moyes AJ, Lewis S, Drayton JP, Tang O, Mehta V, Zachary IC, Abraham DJ, Tsui J, Hobbs AJ. 2019; Endothelial C-type natriuretic peptide is a critical regulator of angiogenesis and vascular remodeling. Circulation. 139:1612–28. DOI: 10.1161/CIRCULATIONAHA.118.036344. PMID: 30586761. PMCID: PMC6438487.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of 12 Autopsy Cases related with Insurance Money
- A Comparative Analysis of the Official Crime Statistics of People with Mental Illness and Public Perception
- A Medicolegal Consideration on Discharge Against Mecical Advice in Korea
- A Clinical Result of PKP performed on the Family with Congenital Hereditary Endothelial Dystrophy
- Endothelial Cell Density Changes in the Normal Korean Cornea During Life