J Korean Med Sci.
2023 Jun;38(25):e193. 10.3346/jkms.2023.38.e193.
Development of Korea Neuroethics Guidelines
- Affiliations
-
- 1Department of Medical Humanities and Ethics, Hanyang University College of Medicine, Seoul, Korea
- 2School of Law/Bioethics Policy Studies, Ewha Womans University, Seoul, Korea
- 3Department of Pre-Medicine, College of Medicine, Ewha Womans University, Seoul, Korea
- 4Department of Criminal Justice Policy Research, Korean Institute of Criminology and Justice, Seoul, Korea
- 5Department of Medical Science, College of Medicine, Catholic Kwandong University, Gangneung, Korea
- 6Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea
- 7Department of Philosophy, Dongguk University, Seoul, Korea
- KMID: 2544009
- DOI: http://doi.org/10.3346/jkms.2023.38.e193
Abstract
- Background
Advances in neuroscience and neurotechnology provide great benefits to humans though unknown challenges may arise. We should address these challenges using new standards as well as existing ones. Novel standards should include ethical, legal, and social aspects which would be appropriate for advancing neuroscience and technology. Therefore, the Korea Neuroethics Guidelines were developed by stakeholders related to neuroscience and neurotechnology, including experts, policy makers, and the public in the Republic of Korea. Method: The guidelines were drafted by neuroethics experts, were disclosed at a public hearing, and were subsequently revised by opinions of various stakeholders.
Results
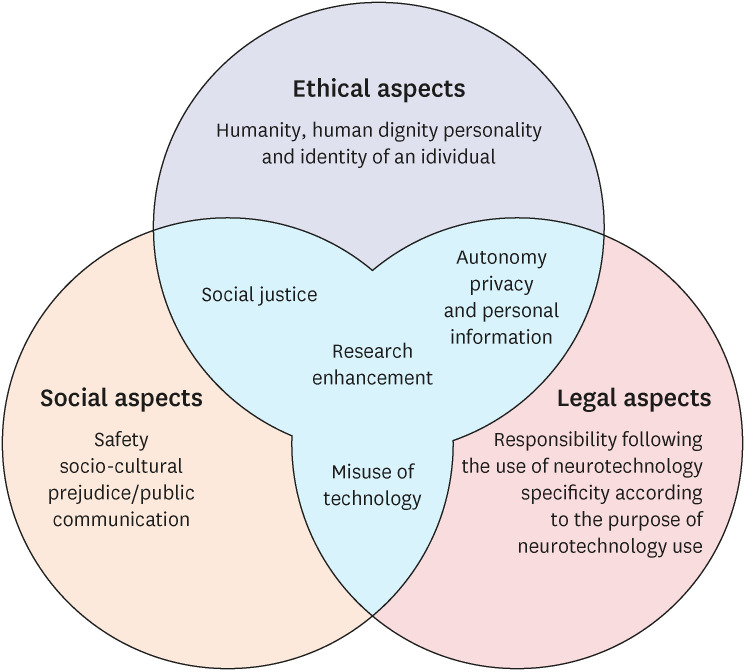
The guidelines are composed of twelve issues; humanity or human dignity, individual personality and identity, social justice, safety, sociocultural prejudice and public communication, misuse of technology, responsibility for the use of neuroscience and technology, specificity according to the purpose of using neurotechnology, autonomy, privacy and personal information, research, and enhancement.
Conclusion
Although the guidelines may require a more detailed discussion after future advances in neuroscience and technology or changes in socio-cultural milieu, the development of the Korea Neuroethics Guidelines is a milestone for the scientific community and society in general for the ongoing development in neuroscience and neurotechnology.
Keyword
Figure
Reference
-
1. Stilgoe J, Owen R, Macnaghten P. Developing a framework for responsible innovation. Res Policy. 2013; 42(9):1568–1580.2. Owen R, Macnaghten P, Stilgoe J. Responsible research and innovation: from science in society to science for society, with society. Sci Public Policy. 2012; 39(6):751–760.3. Salles A, Evers K, Farisco M. Neuroethics and philosophy in responsible research and innovation: the case of the Human Brain Project. Neuroethics. 2019; 12(2):201–211.4. Lee S, Uh S, Li Z. Existing Neuroethics Guidelines. Global Neuroethics Summit 2020.5. Zimmer A. A Neuroethics Intergration Landscape Report. Global Neuroethics Summit 2020-2021.6. Ogbogu U, Ahmed N. Ethical, Legal, and Social Implications (ELSI) research: methods and approaches. Curr Protoc. 2022; 2(1):e354. PMID: 35041252.7. OECD. OECD Recommendation on Responsible Innovation in Neurotechnology. Updated 2019. Accessed May 2, 2022. https://www.oecd.org/science/recommendation-on-responsible-innovation-in-neurotechnology.htm .8. Nuffield Council on Bioethics. Novel Technologies: Intervening in the Brain. London, UK: Nuffield Council on Bioethics;2013.9. Presidential Commission for the Study of Bioethical Issues. Gray Matters: Topics at the Intersection of Neuroscience, Ethics, and Society, Volume II. Updated 2015. Accessed April 20, 2022. https://bioethicsarchive.georgetown.edu/pcsbi/sites/default/files/GrayMatter_V2_508.pdf .10. Greely HT, Grady C, Ramos KM, Chiong W, Eberwine J, Farahany NA, et al. Neuroethics guiding principles for the NIH BRAIN Initiative. J Neurosci. 2018; 38(50):10586–10588. PMID: 30541767.11. Nam SM, Choi MY. Major issues and prospects of neuroethics. Focusing on the analysis of existing neuroethical guidelines. Asia Pac J Health Law Ethics. 2022; 15(3):1–30.12. Cinel C, Valeriani D, Poli R. Neurotechnologies for human cognitive augmentation: current state of the art and future prospects. Front Hum Neurosci. 2019; 13:13. PMID: 30766483.13. Hescham S, Liu H, Jahanshahi A, Temel Y. Deep brain stimulation and cognition: translational aspects. Neurobiol Learn Mem. 2020; 174:107283. PMID: 32739395.14. Wu Y, Mo J, Sui L, Zhang J, Hu W, Zhang C, et al. Deep brain stimulation in treatment-resistant depression: a systematic review and meta-analysis on efficacy and safety. Front Neurosci. 2021; 15:655412. PMID: 33867929.15. Chang CH, Chen SY, Hsiao YL, Tsai ST, Tsai HC. Hypomania with hypersexuality following bilateral anterior limb stimulation in obsessive-compulsive disorder. J Neurosurg. 2010; 112(6):1299–1300. PMID: 19911886.16. Gilbert F, Goddard E, Viaña JNM, Carter A, Horne M. I miss being me: phenomenological effects of deep brain stimulation. AJOB Neurosci. 2017; 8(2):96–109.17. Goering S, Brown T, Klein E. Neurotechnology ethics and relational agency. Philos Compass. 2021; 16(4):e12734. PMID: 34531923.18. Stieglitz T. Of man and mice: translational research in neurotechnology. Neuron. 2020; 105(1):12–15. PMID: 31951526.19. Kaebnick GE, Heitman E, Collins JP, Delborne JA, Landis WG, Sawyer K, et al. Precaution and governance of emerging technologies. Science. 2016; 354(6313):710–711. PMID: 27846595.20. Garden H, Bowman DM, Haesler S, Winickoff DE. Neurotechnology and society: strengthening responsible innovation in brain science. Neuron. 2016; 92(3):642–646. PMID: 27810009.21. Illes J, Blakemore C, Hansson MG, Hensch TK, Leshner A, Maestre G, et al. International perspectives on engaging the public in neuroethics. Nat Rev Neurosci. 2005; 6(12):977–982. PMID: 16340957.22. Illes J, Moser MA, McCormick JB, Racine E, Blakeslee S, Caplan A, et al. Neurotalk: improving the communication of neuroscience research. Nat Rev Neurosci. 2010; 11(1):61–69. PMID: 19953102.23. McCoy LG, Brenna C, Morgado F, Chen S, Das S. Neuroethics, neuroscience, and the project of human self-understanding. AJOB Neurosci. 2020; 11(3):207–209. PMID: 34029491.24. Global Neuroethics Summit Delegates. Rommelfanger KS, Jeong SJ, Ema A, Fukushi T, Kasai K, et al. Neuroethics questions to guide ethical research in the international brain initiatives. Neuron. 2018; 100(1):19–36. PMID: 30308169.25. Choi MY, Kim CS. Criminal Law Issues Regarding Surgeries Using Surgical Robots. Seoul, Korea: Korean Institute of Criminology and Justice;2017.26. Kim JY. Medical Disputes and Law. 2nd ed. Seoul, Korea: Yulgok Publishing Company;2015.27. Schneider L. Neue Behandlungsmethoden im Arzthaftungsrecht. Berlin, German: Springer;2010.28. Schmitz-Luhn B, Katzenmeier C, Woopen C. Law and ethics of deep brain stimulation. Int J Law Psychiatry. 2012; 35(2):130–136. PMID: 22244083.29. Desmoulin-Canselier S. Ethical and legal issues in deep brain stimulation: an overview. D’Aloia A, Errigo MC, editors. Neuroscience and Law: Complicated Crossings and New Perspectives. Cham, Switzerland: Springer;2020. p. 319–337.30. Glannon W. Stimulating brains, altering minds. J Med Ethics. 2009; 35(5):289–292. PMID: 19407032.31. Wexler A. A pragmatic analysis of the regulation of consumer transcranial direct current stimulation (TDCS) devices in the United States. J Law Biosci. 2015; 2(3):669–696. PMID: 27774217.32. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices (Medical Device Regulation, MDR), Article 1, Number 2.33. Bae JD. General Principles of Criminal Law. 15th ed. Seoul, Korea: Hongmunsa;2020.34. Lavazza A. Free will and autonomy in the age of neurotechnologies. Lopez-Silva P, Valera L, editors. Protecting the Mind. Cham, Switzerland: Springer;2022. p. 41–58.35. Parastarfeizabadi M, Kouzani AZ. Advances in closed-loop deep brain stimulation devices. J Neuroeng Rehabil. 2017; 14(1):79. PMID: 28800738.36. Rainey S, Martin S, Christen A, Mégevand P, Fourneret E. Brain recording, mind-reading, and neurotechnology: ethical issues from consumer devices to brain-based speech decoding. Sci Eng Ethics. 2020; 26(4):2295–2311. PMID: 32356091.37. Ienca M. Neuroprivacy, neurosecurity and brain-hacking: emerging issues in neural engineering. Bioethica Forum. 2015; 8(2):51–53.38. Kreitmair KV. Dimensions of ethical direct-to-consumer neurotechnologies. AJOB Neurosci. 2019; 10(4):152–166. PMID: 31642755.39. Lee EY. A study on the improvement of legislation regarding clinical trials. Northeast Asian Law Journal. 2020; 13(3):163–184.40. Parens E. What does enhancement mean?. Parens E, editor. Enhancing Human Traits: Ethical and Social Implications. Washington D.C., USA: Georgetown University Press;1998.41. Lenk C. Therapie und Enhancement: Ziele und Grenzen der modernen Medizin. Muenster, Germany: LIT Verlag;2002.42. Buchanan A. Better Than Human: The Promise and Perils of Enhancing Ourselves. New York, NY, USA: Oxford University Press;2011.43. Schöne-Seifert B, Talbot D, Opolka U, Ach JS. Neuro-Enhancement: Ethik vor neuen Herausforderungen. Paderborn, Germany: Mentis;2009.44. Knoepffler N, Savulescu J. Der neue Mensche?: Enhancement und Genetik. München, Germany: Verlag Karl Alber;2009.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Policy Analysis for Implementing Neuroethics in Korea’s Brain Research Promotion Act
- An XYZ-axis Matrix Approach for the Integration of Neuroscience and Neuroethics
- Development and Implementation of Clinical Practice Guidelines: Current Status in Korea
- Sentience and Moral Status: Comparative Study on Antonio Damasio\'s Feeling and Nicholas Humphrey\'s Sentience
- Clinical Guidelines for the Treatment and Prevention of Opportunistic Infections in HIV-infected Koreans