J Korean Med Sci.
2023 Jun;38(25):e189. 10.3346/jkms.2023.38.e189.
Antibiotic Prescription in Patients With Coronavirus Disease 2019: Analysis of National Health Insurance System Data in the Republic of Korea
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Artificial Intelligence and Big-Data Convergence Centre, Gil Medical Centre, Gachon University College of Medicine, Incheon, Korea
- 4Department of Preventive Medicine, Gachon University College of Medicine, Incheon, Korea
- 5Division of Infectious Diseases, Department of Internal Medicine, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea
- KMID: 2544007
- DOI: http://doi.org/10.3346/jkms.2023.38.e189
Abstract
- Background
Although coronavirus disease 2019 (COVID-19) is a viral infection, antibiotics are often prescribed due to concerns about accompanying bacterial infection. Therefore, we aimed to analyze the number of patients with COVID-19 who received antibiotic prescriptions, as well as factors that influenced antibiotics prescription, using the National Health Insurance System database.
Methods
We retrospectively reviewed claims data for adults aged ≥ 19 years hospitalized for COVID-19 from December 1, 2019 to December 31, 2020. According to the National Institutes of Health guidelines for severity classification, we calculated the proportion of patients who received antibiotics and the number of days of therapy per 1,000 patient-days. Factors contributing to antibiotic use were determined using linear regression analysis. In addition, antibiotic prescription data for patients with influenza hospitalized from 2018 to 2021 were compared with those for patients with COVID-19, using an integrated database from Korea Disease Control and Prevention Agency-COVID19-National Health Insurance Service cohort (K-COV-N cohort), which was partially adjusted and obtained from October 2020 to December 2021.
Results
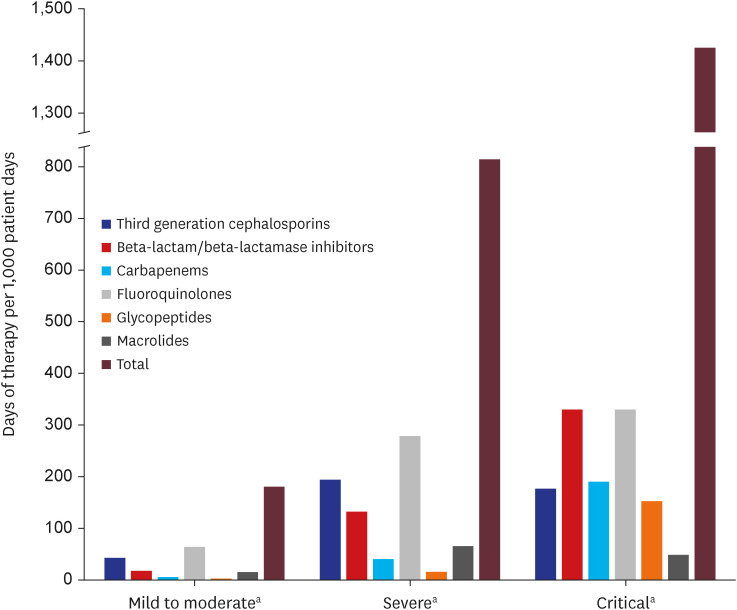
Of the 55,228 patients, 46.6% were males, 55.9% were aged ≥ 50 years, and most patients (88.7%) had no underlying diseases. The majority (84.3%; n = 46,576) were classified as having mild-to-moderate illness, with 11.2% (n = 6,168) and 4.5% (n = 2,484) having severe and critical illness, respectively. Antibiotics were prescribed to 27.3% (n = 15,081) of the total study population, and to 73.8%, 87.6%, and 17.9% of patients with severe, critical, and mild-to-moderate illness, respectively. Fluoroquinolones were the most commonly prescribed antibiotics (15.1%; n = 8,348), followed by third-generation cephalosporins (10.4%; n = 5,729) and beta-lactam/beta-lactamase inhibitors (6.9%; n = 3,822). Older age, COVID-19 severity, and underlying medical conditions contributed significantly to antibiotic prescription requirement. The antibiotic use rate was higher in the influenza group (57.1%) than in the total COVID-19 patient group (21.2%), and higher in severe-to-critical COVID-19 cases (66.6%) than in influenza cases.
Conclusion
Although most patients with COVID-19 had mild to moderate illness, more than a quarter were prescribed antibiotics. Judicious use of antibiotics is necessary for patients with COVID-19, considering the severity of disease and risk of bacterial co-infection.
Figure
Reference
-
1. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020; 35(13):e142. PMID: 32242348.2. Kim M, Yoo JR, Heo ST, Lee HR, Oh H. Clinical characteristics and risk factors for severe disease of coronavirus disease 2019 in a low case fatality rate region in Korea. Infect Chemother. 2021; 53(4):718–729. PMID: 34951535.3. Rawson TM, Moore LS, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020; 71(9):2459–2468. PMID: 32358954.4. Langford BJ, So M, Raybardhan S, Leung V, Soucy JR, Westwood D, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021; 27(4):520–531. PMID: 33418017.5. Stevens RW, Jensen K, O’Horo JC, Shah A. Antimicrobial prescribing practices at a tertiary-care center in patients diagnosed with COVID-19 across the continuum of care. Infect Control Hosp Epidemiol. 2021; 42(1):89–92. PMID: 32703323.6. Westblade LF, Simon MS, Satlin MJ. Bacterial coinfections in coronavirus disease 2019. Trends Microbiol. 2021; 29(10):930–941. PMID: 33934980.7. Getahun H, Smith I, Trivedi K, Paulin S, Balkhy HH. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull World Health Organ. 2020; 98(7):442–442A. PMID: 32742026.8. Mason JW. Antimicrobials and QT prolongation. J Antimicrob Chemother. 2017; 72(5):1272–1274. PMID: 28160473.9. Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016; 387(10014):176–187. PMID: 26603922.10. Kyoung DS, Kim HS. Understanding and utilizing claim data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) database for research. J Lipid Atheroscler. 2022; 11(2):103–110. PMID: 35656154.11. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization;1993. p. 86–92.12. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011; 173(6):676–682. PMID: 21330339.13. National Institutes of Health, COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. Updated 2022. Accessed December 25, 2022. Available from: https://www.covid19treatmentguidelines.nih.gov/.14. Akoglu H. User’s guide to correlation coefficients. Turk J Emerg Med. 2018; 18(3):91–93. PMID: 30191186.15. Sen PK. Estimates of the regression coefficient based on Kendall’s tau. J Am Stat Assoc. 1968; 63(324):1379–1389.16. Falcone M, Tiseo G, Giordano C, Leonildi A, Menichini M, Vecchione A, et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021; 76(4):1078–1084. PMID: 33374002.17. Kim SB, Ryoo S, Huh K, Joo EJ, Kim YJ, Choi WS, et al. Revised Korean Society of Infectious Diseases/National Evidence-Based Healthcarea collaborating agency guidelines on the treatment of patients with COVID-19. Infect Chemother. 2021; 53(1):166–219. PMID: 34409790.18. Lee C, Ahn MY, Byeon K, Choi JP, Hahm C, Kim H, et al. Clinical experience with use of remdesivir in the treatment of severe acute respiratory syndrome coronavirus 2: a case series. Infect Chemother. 2020; 52(3):369–380. PMID: 32757500.19. Joo EJ, Ko JH, Kim SE, Kang SJ, Baek JH, Heo EY, et al. Clinical and virologic effectiveness of remdesivir treatment for severe coronavirus disease 2019 (COVID-19) in Korea: a nationwide multicenter retrospective cohort study. J Korean Med Sci. 2021; 36(11):e83. PMID: 33754512.20. Park DH, Kang CK, Choe PG, Kim NJ, Park WB, Oh MD. How we have treated severe to critically ill patients with coronavirus disease 2019 in Korea. J Korean Med Sci. 2022; 37(49):e353. PMID: 36536547.21. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229):1054–1062. PMID: 32171076.22. He S, Liu W, Jiang M, Huang P, Xiang Z, Deng D, et al. Clinical characteristics of COVID-19 patients with clinically diagnosed bacterial co-infection: a multi-center study. PLoS One. 2021; 16(4):e0249668. PMID: 33819304.23. Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020; 26(12):1622–1629. PMID: 32711058.24. Pasero D, Cossu AP, Terragni P. Multi-drug resistance bacterial infections in critically ill patients admitted with COVID-19. Microorganisms. 2021; 9(8):1773. PMID: 34442852.25. Park DH, Lee CM, Chang E, Kang CK, Park WB, Kim NJ, et al. Clinical impact of empirical antibiotic therapy in patients with coronavirus disease 2019 requiring oxygen therapy. J Korean Med Sci. 2022; 37(29):e238. PMID: 35880508.26. Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020; 56(2):106051. PMID: 32534186.27. Vazzana N, Dipaola F, Ognibene S. Procalcitonin and secondary bacterial infections in COVID-19: association with disease severity and outcomes. Acta Clin Belg. 2022; 77(2):268–272. PMID: 32966166.28. Carbonell R, Urgelés S, Salgado M, Rodríguez A, Reyes LF, Fuentes YV, et al. Negative predictive value of procalcitonin to rule out bacterial respiratory co-infection in critical covid-19 patients. J Infect. 2022; 85(4):374–381. PMID: 35781017.29. Pandey M, May A, Tan L, Hughes H, Jones JP, Harrison W, et al. Comparative incidence of early and late bloodstream and respiratory tract co-infection in patients admitted to ICU with COVID-19 pneumonia versus Influenza A or B pneumonia versus no viral pneumonia: Wales multicentre ICU cohort study. Crit Care. 2022; 26(1):158. PMID: 35655224.30. Kim HS, Kim JH, Shin SY, Kang YA, Lee HG, Kim JS, et al. Fatal cases of 2009 pandemic influenza A (H1N1) in Korea. J Korean Med Sci. 2011; 26(1):22–27. PMID: 21218025.31. Byeon KH, Kim J, Choi BY, Kim JY, Lee N. Factors affecting the incidence of hospitalized pneumonia after influenza infection in Korea using the national health insurance research database, 2014–2018: focusing on the effect of antiviral therapy in the 2017 flu season. J Korean Med Sci. 2020; 35(38):e318. PMID: 32989929.32. Lim JK, Kim TH, Kilgore PE, Aiello AE, Choi BM, Lee KC, et al. The association between influenza treatment and hospitalization-associated outcomes among Korean children with laboratory-confirmed influenza. J Korean Med Sci. 2014; 29(4):485–493. PMID: 24753694.33. Sohn KM, Chung DR, Baek JY, Kim SH, Joo EJ, Ha YE, et al. Post-influenza pneumonia caused by the USA300 community-associated methicillin-resistant Staphylococcus aureus in Korea. J Korean Med Sci. 2012; 27(3):313–316. PMID: 22379344.34. Joseph C, Togawa Y, Shindo N. Bacterial and viral infections associated with influenza. Influenza Other Respi Viruses. 2013; 7:Suppl 2. (Suppl 2):105–113.35. Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017; 8:1041. PMID: 28690590.36. Korea Disease Control and Prevention Agency. Coronavirus (COVID-19), Republic of Korea. Updated 2022. Accessed December 25, 2022. https://ncov.kdca.go.kr/ .37. Lai CC, Chen SY, Ko WC, Hsueh PR. Increased antimicrobial resistance during the COVID-19 pandemic. Int J Antimicrob Agents. 2021; 57(4):106324. PMID: 33746045.38. Jeon K, Jeong S, Lee N, Park MJ, Song W, Kim HS, et al. Impact of COVID-19 on antimicrobial consumption and spread of multidrug-resistance in bacterial infections. Antibiotics (Basel). 2022; 11(4):535. PMID: 35453286.39. Segala FV, Bavaro DF, Di Gennaro F, Salvati F, Marotta C, Saracino A, et al. Impact of SARS-CoV-2 epidemic on antimicrobial resistance: a literature review. Viruses. 2021; 13(11):2110. PMID: 34834917.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antibiotic Use in Korean Children Diagnosed With Acute Bronchiolitis: Analysis of the National Health Insurance Reimbursement Data
- Analysis of Dental Antibiotic Prescriptions for Children and Adolescents in South Korea
- Outpatient Antibiotic Prescription Patterns for Respiratory Tract Infections of Infants
- The Trend of Acute Respiratory Tract Infections and Antibiotic Prescription Rates in Outpatient Settings using Health Insurance Data
- Comparative Analysis of Prescription for Splitted Tablet using the HIRA-NPS (Health Insurance Review & Assessment Service-National Patient Sample)