J Korean Neurosurg Soc.
2023 Jul;66(4):400-408. 10.3340/jkns.2022.0115.
Nicotinamide Mononucleotide Adenylyl Transferase 2 Inhibition Aggravates Neurological Damage after Traumatic Brain Injury in a Rat Model
- Affiliations
-
- 1Department of Intensive Care Unit, The Affiliated Zhangjiagang Hospital of Soochow University, Suzhou, China
- 2Department of Neurosurgery, The Affiliated Zhangjiagang Hospital of Soochow University, Suzhou, China
- 3Department of Neurobiology, The Affiliated Xuzhou Medical University, XuZhou, China
- 4Department of Orthopedics, The Affiliated Zhangjiagang Hospital of Soochow University, Suzhou, China
- KMID: 2543531
- DOI: http://doi.org/10.3340/jkns.2022.0115
Abstract
Objective
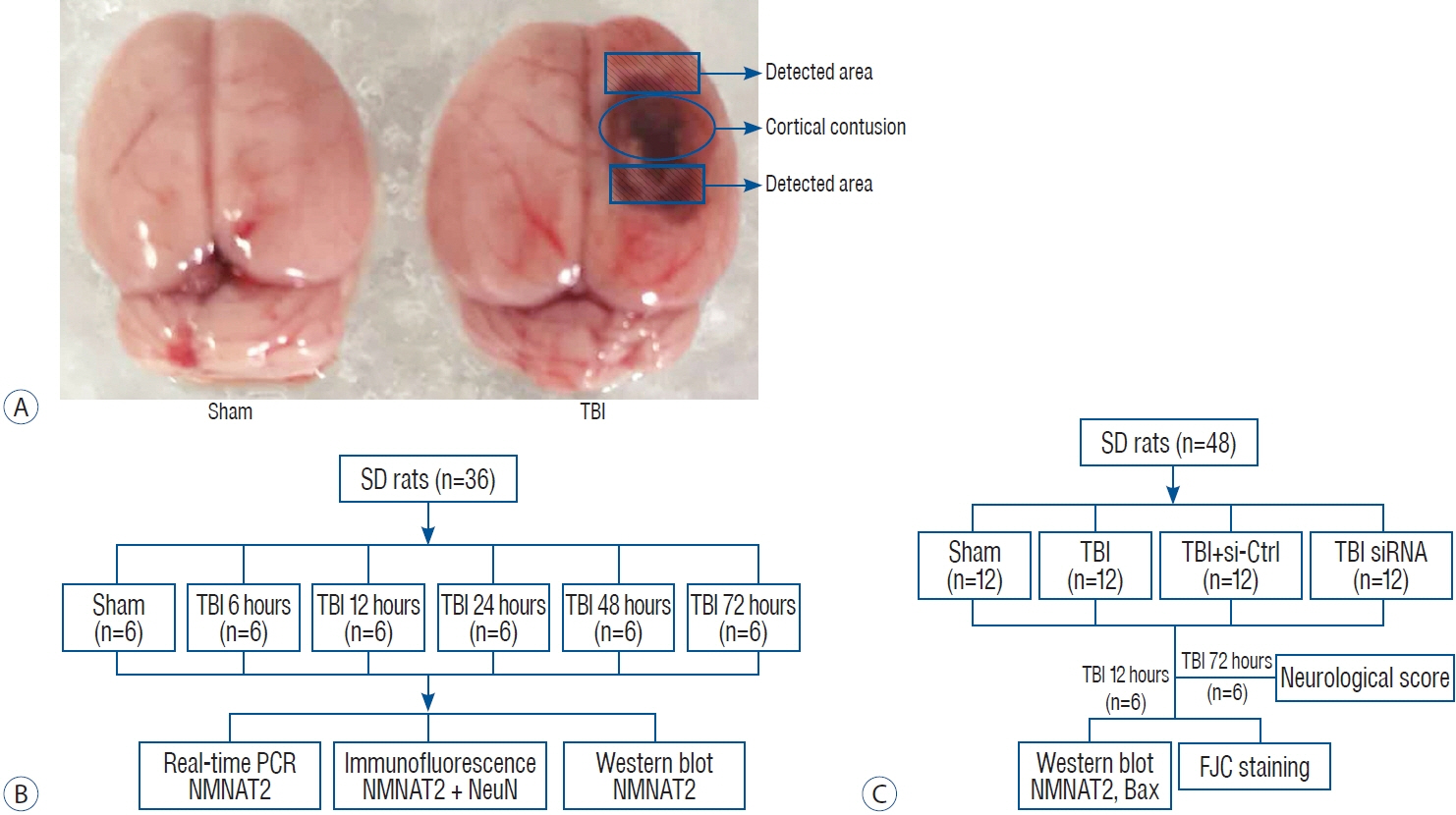
: Nicotinamide mononucleotide adenylyl transferase 2 (NMNAT2) is a crucial factor for the survival of neuron. The role of NMNAT2 in damage following traumatic brain injury (TBI) remains unknown. This study was designed to investigate the role of NMNAT2 in TBI-induced neuronal degeneration and neurological deficits in rats.
Methods
: The TBI model was established in Sprague-Dawley rats by a weight-dropping method. Real-time polymerase chain reaction, western blot, immunofluorescence, Fluoro-Jade C staining, and neurological score analyses were carried out.
Results
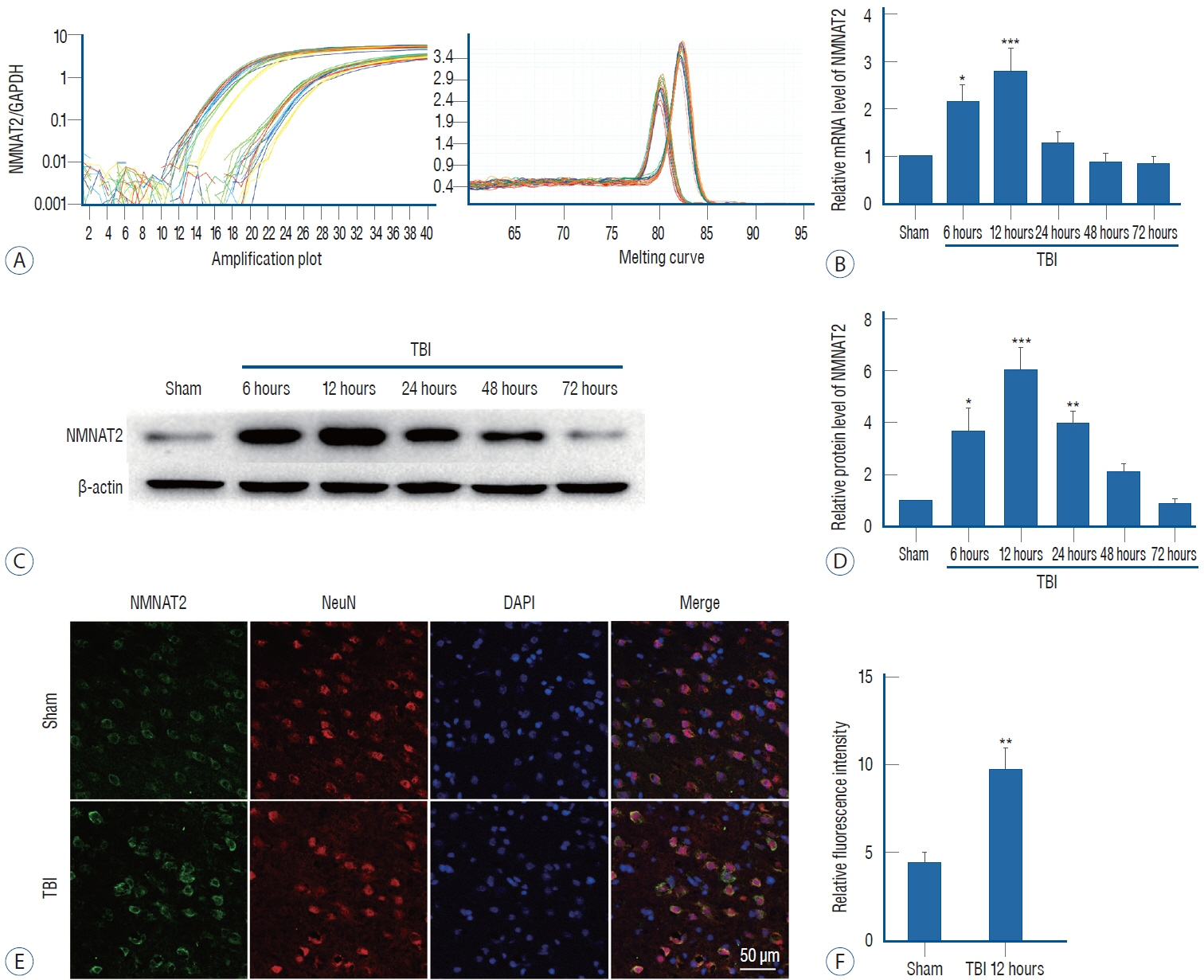
: NMNAT2 mRNA and protein levels were increased in the injured-side cortex at 6 hours and peaked 12 hours after TBI. Knocking down NMNAT2 with an injection of small interfering RNA in lateral ventricle significantly exacerbated neuronal degeneration and neurological deficits after TBI, which were accompanied by increased expression of BCL-2-associated X protein (Bax).
Conclusion
: NMNAT2 expression is increased and NMNAT2 exhibits neuroprotective activity in the early stages after TBI, and Bax signaling pathway may be involved in the process. Thus, NMNAT2 is likely to be an important target to prevent secondary damage following TBI.
Keyword
Figure
Reference
-
References
1. Autret A, Martin SJ. Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell. 36:355–363. 2009.
Article2. Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep. 3:1422–1429. 2013.3. Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 280:36334–36341. 2005.
Article4. Cai Y, Yu SS, Chen SR, Pi RB, Gao S, Li H, et al. Nmnat2 protects cardiomyocytes from hypertrophy via activation of SIRT6. FEBS Lett. 586:866–874. 2012.
Article5. Carteri RB, Kopczynski A, Rodolphi MS, Strogulski NR, Sartor M, Feldmann M, et al. Testosterone administration after traumatic brain injury reduces mitochondrial dysfunction and neurodegeneration. J Neurotrauma. 36:2246–2259. 2019.
Article6. Ding C, Hammarlund M. Mechanisms of injury-induced axon degeneration. Curr Opin Neurobiol. 57:171–178. 2019.
Article7. Ekert PG, Read SH, Silke J, Marsden VS, Kaufmann H, Hawkins CJ, et al. Apaf-1 and caspase-9 accelerate apoptosis, but do not determine whether factor-deprived or drug-treated cells die. J Cell Biol. 165:835–842. 2004.
Article8. Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science. 348:453–457. 2015.
Article9. Gerdts J, Summers DW, Milbrandt J, DiAntonio A. Axon self-destruction: new links among SARM1, MAPKs, and NAD+ metabolism. Neuron. 89:449–460. 2016.
Article10. Henninger N, Bouley J, Sikoglu EM, An J, Moore CM, King JA, et al. Attenuated traumatic axonal injury and improved functional outcome after traumatic brain injury in mice lacking Sarm1. Brain. 139:1094–1105. 2016.
Article11. Huang J, Tang D, Cao Y, Wang Y, Long J, Wei L, et al. Inhibition of PDE10A-rescued TBI-induced neuroinflammation and apoptosis through the cAMP/PKA/NLRP3 pathway. Evid Based Complement Alternat Med. 2022:3311250. 2022.
Article12. Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 22:341–353. 2007.
Article13. Jayaram HN, Kusumanchi P, Yalowitz JA. NMNAT expression and its relation to NAD metabolism. Curr Med Chem. 18:1962–1972. 2011.
Article14. Jennings JS, Gerber AM, Vallano ML. Pharmacological strategies for neuroprotection in traumatic brain injury. Mini Rev Med Chem. 8:689–701. 2008.
Article15. Lau C, Niere M, Ziegler M. The NMN/NaMN adenylyltransferase (NMNAT) protein family. Front Biosci (Landmark Ed). 14:410–431. 2009.
Article16. Li D, Ni H, Rui Q, Gao R, Chen G. Deletion of Mst1 attenuates neuronal loss and improves neurological impairment in a rat model of traumatic brain injury. Brain Res. 1688:15–21. 2018.
Article17. Loreto A, Di Stefano M, Gering M, Conforti L. Wallerian degeneration is executed by an NMN-SARM1-dependent late Ca(2+) influx but only modestly influenced by mitochondria. Cell Rep. 13:2539–2552. 2015.
Article18. Menon DK, Schwab K, Wright DW, Maas AI; Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: definition of traumatic brain injury. Arch Phys Med Rehabil. 91:1637–1640. 2010.
Article19. Mete M, Aydemir I, Unsal UU, Collu F, Vatandas G, Gurcu B, et al. Neuroprotective effects of oleocanthal, a compound in virgin olive oil, in a rat model of traumatic brain injury. Turk Neurosurg. 28:858–865. 2018.
Article20. Mouchiroud L, Houtkooper RH, Auwerx J. NAD+ metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol. 48:397–408. 2013.21. Orsomando G, Cialabrini L, Amici A, Mazzola F, Ruggieri S, Conforti L, et al. Simultaneous single-sample determination of NMNAT isozyme activities in mouse tissues. PLoS One. 7:e53271. 2012.
Article22. Sabirzhanov B, Faden AI, Aubrecht T, Henry R, Glaser E, Stoica BA. MicroRNA-711-induced downregulation of angiopoietin-1 mediates neuronal cell death. J Neurotrauma. 35:2462–2481. 2018.
Article23. Sabirzhanov B, Zhao Z, Stoica BA, Loane DJ, Wu J, Borroto C, et al. Downregulation of miR-23a and miR-27a following experimental traumatic brain injury induces neuronal cell death through activation of proapoptotic Bcl-2 proteins. J Neurosci. 34:10055–10071. 2014.
Article24. Shen H, Chen Z, Wang Y, Gao A, Li H, Cui Y, et al. Role of neurexin-1ß and neuroligin-1 in cognitive dysfunction after subarachnoid hemorrhage in rats. Stroke. 46:2607–2615. 2015.
Article25. Summers DW, Gibson DA, DiAntonio A, Milbrandt J. SARM1-specific motifs in the TIR domain enable NAD+ loss and regulate injury-induced SARM1 activation. Proc Natl Acad Sci U S A. 113:E6271–E6280. 2016.26. Wang Y, Gao A, Xu X, Dang B, You W, Li H, et al. The neuroprotection of lysosomotropic agents in experimental subarachnoid hemorrhage probably involving the apoptosis pathway triggering by cathepsins via chelating intralysosomal iron. Mol Neurobiol. 52:64–77. 2015.
Article27. Wang Y, Liu Y, Lopez D, Lee M, Dayal S, Hurtado A, et al. Protection against TBI-induced neuronal death with post-treatment with a selective calpain-2 inhibitor in mice. J Neurotrauma. 35:105–117. 2018.
Article28. Yamagishi Y, Tessier-Lavigne M. An atypical SCF-like ubiquitin ligase complex promotes wallerian degeneration through regulation of axonal Nmnat2. Cell Rep. 17:774–782. 2016.
Article29. Yang J, Wu Z, Renier N, Simon DJ, Uryu K, Park DS, et al. Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell. 160:161–176. 2015.
Article30. Zhao Z, Zhou Y, Tian Y, Li M, Dong JF, Zhang J. Cellular microparticles and pathophysiology of traumatic brain injury. Protein Cell. 8:801–810. 2017.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Diagnostic Criteria of Mild Traumatic Brain Injury
- Animal Model of Traumatic Brain Injury Induced by Controlled Cortical Impact Device
- Nicotinamide Reduces the Infarct Volume in a Rat Model of Transient Middle Cerebral Artery Occlusion
- Animal Models of Traumatic Brain Injury
- Post-Traumatic Cerebral Infarction Following Low-Energy Penetrating Craniocerebral Injury Caused by a Nail