Korean Circ J.
2023 Jun;53(6):367-386. 10.4070/kcj.2023.0098.
Are There Hopeful Therapeutic Strategies to Regenerate the Infarcted Hearts?
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Seoul St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 2Division of Cardiology, Department of Internal Medicine, Eunpyeong St. Mary’s Hospital, The Catholic University of Korea, Seoul, Korea
- 3Department of Biomedicine & Health Sciences, The Catholic University of Korea, Seoul, Korea
- 4Department of Biomedical Sciences, City University of Hong Kong, Kowloon, Hong Kong
- KMID: 2543050
- DOI: http://doi.org/10.4070/kcj.2023.0098
Abstract
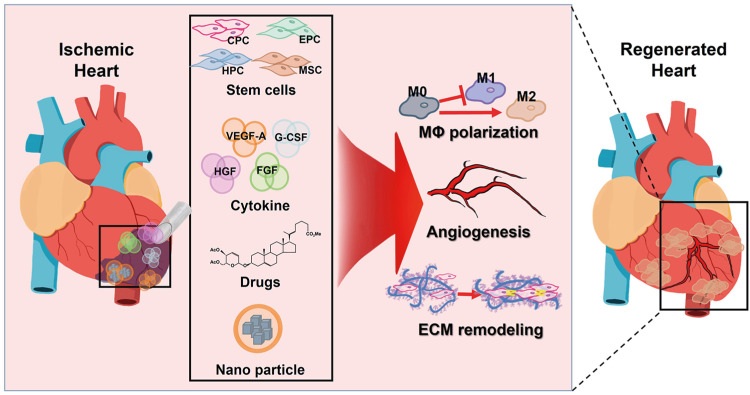
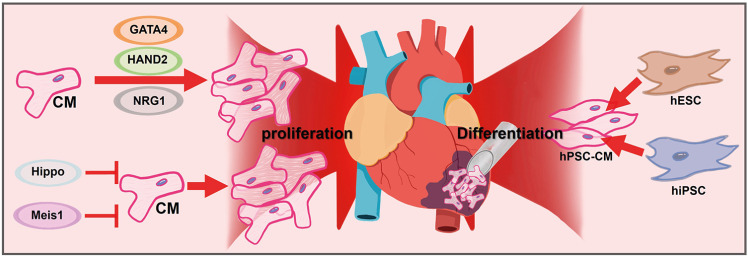
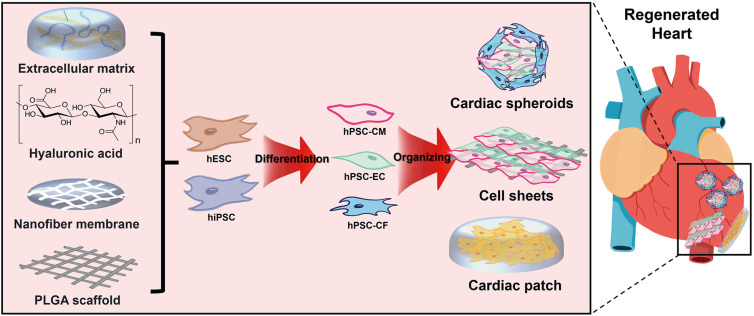
- Ischemic heart disease remains the primary cause of morbidity and mortality worldwide. Despite significant advancements in pharmacological and revascularization techniques in the late 20th century, heart failure prevalence after myocardial infarction has gradually increased over the last 2 decades. After ischemic injury, pathological remodeling results in cardiomyocytes (CMs) loss and fibrosis, which leads to impaired heart function. Unfortunately, there are no clinical therapies to regenerate CMs to date, and the adult heart’s limited turnover rate of CMs hinders its ability to self-regenerate. In this review, we present novel therapeutic strategies to regenerate injured myocardium, including (1) reconstruction of cardiac niche microenvironment, (2) recruitment of functional CMs by promoting their proliferation or differentiation, and (3) organizing 3-dimensional tissue construct beyond the CMs. Additionally, we highlight recent mechanistic insights that govern these strategies and identify current challenges in translating these approaches to human patients.
Keyword
Figure
Reference
-
1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020; 396:1204–1222. PMID: 33069326.2. Jenča D, Melenovský V, Stehlik J, et al. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. 2021; 8:222–237. PMID: 33319509.3. Bhar-Amato J, Davies W, Agarwal S. Ventricular arrhythmia after acute myocardial infarction: ‘the perfect storm’. Arrhythm Electrophysiol Rev. 2017; 6:134–139. PMID: 29018522.4. Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010; 56:254–263. PMID: 20633817.5. Menasché P, Hagège AA, Scorsin M, et al. Myoblast transplantation for heart failure. Lancet. 2001; 357:279–280. PMID: 11214133.6. Banerjee MN, Bolli R, Hare JM. Clinical studies of cell therapy in cardiovascular medicine: recent developments and future directions. Circ Res. 2018; 123:266–287. PMID: 29976692.7. Wang Y, Wei J, Zhang P, et al. Neuregulin-1, a potential therapeutic target for cardiac repair. Front Pharmacol. 2022; 13:945206. PMID: 36120374.8. Li Q, Li B, Wang X, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997; 100:1991–1999. PMID: 9329962.9. Räsänen M, Sultan I, Paech J, et al. VEGF-B promotes endocardium-derived coronary vessel development and cardiac regeneration. Circulation. 2021; 143:65–77. PMID: 33203221.10. Gwathmey JK, Yerevanian A, Hajjar RJ. Targeting sarcoplasmic reticulum calcium ATPase by gene therapy. Hum Gene Ther. 2013; 24:937–947. PMID: 24164241.11. Lee CS, Kim J, Cho HJ, Kim HS. Cardiovascular regeneration via stem cells and direct reprogramming: a review. Korean Circ J. 2022; 52:341–353. PMID: 35502566.12. Bui TV, Hwang JW, Lee JH, Park HJ, Ban K. Challenges and limitations of strategies to promote therapeutic potential of human mesenchymal stem cells for cell-based cardiac repair. Korean Circ J. 2021; 51:97–113. PMID: 33525065.13. Leri A, Rota M, Hosoda T, Goichberg P, Anversa P. Cardiac stem cell niches. Stem Cell Res. 2014; 13:631–646. PMID: 25267073.14. Pries AR, Reglin B. Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur Heart J. 2017; 38:478–488. PMID: 26843279.15. Rajendran P, Rengarajan T, Thangavel J, et al. The vascular endothelium and human diseases. Int J Biol Sci. 2013; 9:1057–1069. PMID: 24250251.16. Soares RO, Losada DM, Jordani MC, Évora P, Castro-E-Silva O. Ischemia/reperfusion injury revisited: an overview of the latest pharmacological strategies. Int J Mol Sci. 2019; 20:5034. PMID: 31614478.17. Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014; 114:565–571. PMID: 24481846.18. van der Laan AM, Piek JJ, van Royen N. Targeting angiogenesis to restore the microcirculation after reperfused MI. Nat Rev Cardiol. 2009; 6:515–523. PMID: 19528962.19. Saeed M, Saloner D, Martin A, et al. Adeno-associated viral vector-encoding vascular endothelial growth factor gene: effect on cardiovascular MR perfusion and infarct resorption measurements in swine. Radiology. 2007; 243:451–460. PMID: 17384240.20. Battler A, Scheinowitz M, Bor A, et al. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol. 1993; 22:2001–2006. PMID: 7504006.21. Chen XH, Minatoguchi S, Kosai K, et al. In vivo hepatocyte growth factor gene transfer reduces myocardial ischemia-reperfusion injury through its multiple actions. J Card Fail. 2007; 13:874–883. PMID: 18068622.22. Saeed M, Martin A, Ursell P, et al. MR assessment of myocardial perfusion, viability, and function after intramyocardial transfer of VM202, a new plasmid human hepatocyte growth factor in ischemic swine myocardium. Radiology. 2008; 249:107–118. PMID: 18682582.23. Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008; 28:208–216. PMID: 17951319.24. Massa M, Rosti V, Ferrario M, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005; 105:199–206. PMID: 15345590.25. Templin C, Kotlarz D, Faulhaber J, et al. Ex vivo expanded hematopoietic progenitor cells improve cardiac function after myocardial infarction: role of beta-catenin transduction and cell dose. J Mol Cell Cardiol. 2008; 45:394–403. PMID: 18671980.26. Halkos ME, Zhao ZQ, Kerendi F, et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008; 103:525–536. PMID: 18704259.27. Schuleri KH, Amado LC, Boyle AJ, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008; 294:H2002–H2011. PMID: 18310523.28. Tang J, Xie Q, Pan G, Wang J, Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006; 30:353–361. PMID: 16829080.29. Kanellakis P, Slater NJ, Du XJ, Bobik A, Curtis DJ. Granulocyte colony-stimulating factor and stem cell factor improve endogenous repair after myocardial infarction. Cardiovasc Res. 2006; 70:117–125. PMID: 16497284.30. Sato T, Suzuki H, Kusuyama T, et al. G-CSF after myocardial infarction accelerates angiogenesis and reduces fibrosis in swine. Int J Cardiol. 2008; 127:166–173. PMID: 17692407.31. Guo Y, He J, Wu J, et al. Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Arch Med Res. 2008; 39:179–188. PMID: 18164961.32. Lu F, Zhao X, Wu J, et al. MSCs transfected with hepatocyte growth factor or vascular endothelial growth factor improve cardiac function in the infarcted porcine heart by increasing angiogenesis and reducing fibrosis. Int J Cardiol. 2013; 167:2524–2532. PMID: 22981278.33. Park BW, Jung SH, Das S, et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci Adv. 2020; 6:eaay6994. PMID: 32284967.34. Park SJ, Kim RY, Park BW, et al. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun. 2019; 10:3123. PMID: 31311935.35. Han C, Nie Y, Lian H, et al. Acute inflammation stimulates a regenerative response in the neonatal mouse heart. Cell Res. 2015; 25:1137–1151. PMID: 26358185.36. Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A. 2013; 110:9415–9420. PMID: 23690624.37. King MW, Neff AW, Mescher AL. The developing Xenopus limb as a model for studies on the balance between inflammation and regeneration. Anat Rec (Hoboken). 2012; 295:1552–1561. PMID: 22933418.38. Silvis MJ, Kaffka Genaamd Dengler SE, Odille CA, et al. Damage-associated molecular patterns in myocardial infarction and heart transplantation: the road to translational success. Front Immunol. 2020; 11:599511. PMID: 33363540.39. Cao DJ, Schiattarella GG, Villalobos E, et al. Cytosolic DNA sensing promotes macrophage transformation and governs myocardial ischemic injury. Circulation. 2018; 137:2613–2634. PMID: 29437120.40. Sandanger Ø, Ranheim T, Vinge LE, et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013; 99:164–174. PMID: 23580606.41. Kratofil RM, Kubes P, Deniset JF. Monocyte conversion during inflammation and injury. Arterioscler Thromb Vasc Biol. 2017; 37:35–42. PMID: 27765768.42. Kim Y, Nurakhayev S, Nurkesh A, Zharkinbekov Z, Saparov A. Macrophage polarization in cardiac tissue repair following myocardial infarction. Int J Mol Sci. 2021; 22:2715. PMID: 33800220.43. Shiraishi M, Shintani Y, Shintani Y, et al. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest. 2016; 126:2151–2166. PMID: 27140396.44. Lee JR, Park BW, Kim J, et al. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv. 2020; 6:eaaz0952. PMID: 32494669.45. Zhang H, Kim H, Park BW, et al. CU06-1004 enhances vascular integrity and improves cardiac remodeling by suppressing edema and inflammation in myocardial ischemia-reperfusion injury. Exp Mol Med. 2022; 54:23–34. PMID: 34997212.46. Su Y, Gao J, Kaur P, Wang Z. Neutrophils and macrophages as targets for development of nanotherapeutics in inflammatory diseases. Pharmaceutics. 2020; 12:1222. PMID: 33348630.47. Forte E, Perkins B, Sintou A, et al. Cross-priming dendritic cells exacerbate immunopathology after ischemic tissue damage in the heart. Circulation. 2021; 143:821–836. PMID: 33297741.48. Bansal SS, Ismahil MA, Goel M, et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation. 2019; 139:206–221. PMID: 30586716.49. Srenathan U, Steel K, Taams LS. IL-17+ CD8+ T cells: differentiation, phenotype and role in inflammatory disease. Immunol Lett. 2016; 178:20–26. PMID: 27173097.50. Bansal SS, Ismahil MA, Goel M, et al. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail. 2017; 10:e003688. PMID: 28242779.51. Choo EH, Lee JH, Park EH, et al. Infarcted myocardium-primed dendritic cells improve remodeling and cardiac function after myocardial infarction by modulating the regulatory T cell and macrophage polarization. Circulation. 2017; 135:1444–1457. PMID: 28174192.52. Rieckmann M, Delgobo M, Gaal C, et al. Myocardial infarction triggers cardioprotective antigen-specific T helper cell responses. J Clin Invest. 2019; 129:4922–4936. PMID: 31408441.53. Weirather J, Hofmann UD, Beyersdorf N, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014; 115:55–67. PMID: 24786398.54. Kalkman EA, van Suylen RJ, van Dijk JP, Saxena PR, Schoemaker RG. Chronic aspirin treatment affects collagen deposition in non-infarcted myocardium during remodeling after coronary artery ligation in the rat. J Mol Cell Cardiol. 1995; 27:2483–2494. PMID: 8596199.55. Lefer AM, Polansky EW. Beneficial effects of ibuprofen in acute myocardial ischemia. Cardiology. 1979; 64:265–279. PMID: 476733.56. Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2014; 16:821–847.57. Gao R, Shi H, Chang S, et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol. 2019; 74:105575. PMID: 31299609.58. Fung TH, Yang KY, Lui KO. An emerging role of regulatory T-cells in cardiovascular repair and regeneration. Theranostics. 2020; 10:8924–8938. PMID: 32802172.59. Wirrig EE, Snarr BS, Chintalapudi MR, et al. Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev Biol. 2007; 310:291–303. PMID: 17822691.60. Bassat E, Mutlak YE, Genzelinakh A, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017; 547:179–184. PMID: 28581497.61. Baehr A, Umansky KB, Bassat E, et al. Agrin promotes coordinated therapeutic processes leading to improved cardiac repair in pigs. Circulation. 2020; 142:868–881. PMID: 32508131.62. Chen Z, Xie J, Hao H, et al. Ablation of periostin inhibits post-infarction myocardial regeneration in neonatal mice mediated by the phosphatidylinositol 3 kinase/glycogen synthase kinase 3β/cyclin D1 signalling pathway. Cardiovasc Res. 2017; 113:620–632. PMID: 28453729.63. Kühn B, del Monte F, Hajjar RJ, et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007; 13:962–969. PMID: 17632525.64. Ladage D, Yaniz-Galende E, Rapti K, et al. Stimulating myocardial regeneration with periostin Peptide in large mammals improves function post-myocardial infarction but increases myocardial fibrosis. PLoS One. 2013; 8:e59656. PMID: 23700403.65. Giacca M, Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther. 2012; 19:622–629. PMID: 22378343.66. Del Monte-Nieto G, Fischer JW, Gorski DJ, Harvey RP, Kovacic JC. Basic biology of extracellular matrix in the cardiovascular system, part 1/4: JACC focus seminar. J Am Coll Cardiol. 2020; 75:2169–2188. PMID: 32354384.67. Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014; 15:786–801. PMID: 25415508.68. Seif-Naraghi SB, Singelyn JM, Salvatore MA, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013; 5:173ra25.69. Singelyn JM, Sundaramurthy P, Johnson TD, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012; 59:751–763. PMID: 22340268.70. Wassenaar JW, Gaetani R, Garcia JJ, et al. Evidence for mechanisms underlying the functional benefits of a myocardial matrix hydrogel for post-MI treatment. J Am Coll Cardiol. 2016; 67:1074–1086. PMID: 26940929.71. Korf-Klingebiel M, Reboll MR, Grote K, et al. Heparan sulfate-editing extracellular sulfatases enhance VEGF bioavailability for ischemic heart repair. Circ Res. 2019; 125:787–801. PMID: 31434553.72. Mukherjee D, Wagh G, Mokalled MH, et al. Ccn2a is an injury-induced matricellular factor that promotes cardiac regeneration in zebrafish. Development. 2021; 148:dev193219. PMID: 33234717.73. Notari M, Ventura-Rubio A, Bedford-Guaus SJ, et al. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci Adv. 2018; 4:eaao5553. PMID: 29732402.74. Perestrelo AR, Silva AC, Oliver-De La Cruz J, et al. Multiscale analysis of extracellular matrix remodeling in the failing heart. Circ Res. 2021; 128:24–38. PMID: 33106094.75. Corbin EA, Vite A, Peyster EG, et al. Tunable and reversible substrate stiffness reveals a dynamic mechanosensitivity of cardiomyocytes. ACS Appl Mater Interfaces. 2019; 11:20603–20614. PMID: 31074953.76. Yahalom-Ronen Y, Rajchman D, Sarig R, Geiger B, Tzahor E. Reduced matrix rigidity promotes neonatal cardiomyocyte dedifferentiation, proliferation and clonal expansion. Elife. 2015; 4:e07455. PMID: 26267307.77. Traverse JH, Henry TD, Dib N, et al. First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl Sci. 2019; 4:659–669. PMID: 31709316.78. Walsh S, Pontén A, Fleischmann BK, Jovinge S. Cardiomyocyte cell cycle control and growth estimation in vivo--an analysis based on cardiomyocyte nuclei. Cardiovasc Res. 2010; 86:365–373. PMID: 20071355.79. Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009; 324:98–102. PMID: 19342590.80. Bergmann O, Zdunek S, Felker A, et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015; 161:1566–1575. PMID: 26073943.81. Porrello ER, Olson EN. A neonatal blueprint for cardiac regeneration. Stem Cell Res. 2014; 13:556–570. PMID: 25108892.82. Kikuchi K, Holdway JE, Werdich AA, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010; 464:601–605. PMID: 20336144.83. Schindler YL, Garske KM, Wang J, et al. Hand2 elevates cardiomyocyte production during zebrafish heart development and regeneration. Development. 2014; 141:3112–3122. PMID: 25038045.84. Muralidhar SA, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nakanishi T, Markwald RR, Baldwin HS, editors. Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology. Tokyo: Springer Tokyo;2016. p. 93–101.85. Pesce M, Burba I, Gambini E, Prandi F, Pompilio G, Capogrossi MC. Endothelial and cardiac progenitors: boosting, conditioning and (re)programming for cardiovascular repair. Pharmacol Ther. 2011; 129:50–61. PMID: 21035506.86. Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011; 138:9–22. PMID: 21138973.87. Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014; 141:1614–1626. PMID: 24715453.88. Zheng M, Jacob J, Hung SH, Wang J. The Hippo pathway in cardiac regeneration and homeostasis: new perspectives for cell-free therapy in the injured heart. Biomolecules. 2020; 10:1024. PMID: 32664346.89. Leach JP, Heallen T, Zhang M, et al. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature. 2017; 550:260–264. PMID: 28976966.90. Lin Z, von Gise A, Zhou P, et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014; 115:354–363. PMID: 24833660.91. Morikawa Y, Heallen T, Leach J, Xiao Y, Martin JF. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature. 2017; 547:227–231. PMID: 28581498.92. Falls DL. Neuregulins: functions, forms, and signaling strategies. Exp Cell Res. 2003; 284:14–30. PMID: 12648463.93. Del Monte-Nieto G, Ramialison M, Adam AA, et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature. 2018; 557:439–445. PMID: 29743679.94. Baliga RR, Pimental DR, Zhao YY, et al. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999; 277:H2026–H2037. PMID: 10564160.95. Reischauer S, Arnaout R, Ramadass R, Stainier DY. Actin binding GFP allows 4D in vivo imaging of myofilament dynamics in the zebrafish heart and the identification of Erbb2 signaling as a remodeling factor of myofibril architecture. Circ Res. 2014; 115:845–856. PMID: 25228389.96. Gemberling M, Karra R, Dickson AL, Poss KD. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife. 2015; 4:e05871. PMID: 25830562.97. de Bakker DE, Bouwman M, Dronkers E, et al. Prrx1b restricts fibrosis and promotes Nrg1-dependent cardiomyocyte proliferation during zebrafish heart regeneration. Development. 2021; 148:dev198937. PMID: 34486669.98. Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009; 138:257–270. PMID: 19632177.99. D’Uva G, Aharonov A, Lauriola M, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015; 17:627–638. PMID: 25848746.100. Aharonov A, Shakked A, Umansky KB, et al. ERBB2 drives YAP activation and EMT-like processes during cardiac regeneration. Nat Cell Biol. 2020; 22:1346–1356. PMID: 33046882.101. Denning C, Borgdorff V, Crutchley J, et al. Cardiomyocytes from human pluripotent stem cells: from laboratory curiosity to industrial biomedical platform. Biochim Biophys Acta. 2016; 1863:1728–1748. PMID: 26524115.102. Shiba Y. Pluripotent stem cells for cardiac regeneration—current status, challenges, and future perspectives—. Circ J. 2020; 84:2129–2135. PMID: 33087630.103. Park SJ, Kim H, Lee S, et al. Effect and application of cryopreserved three-dimensional microcardiac spheroids in myocardial infarction therapy. Clin Transl Med. 2022; 12:e721. PMID: 35092703.104. Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014; 510:273–277. PMID: 24776797.105. Liu YW, Chen B, Yang X, et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018; 36:597–605. PMID: 29969440.106. Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. 2015; 142:3113–3125. PMID: 26395140.107. Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol. 2016; 18:246–254. PMID: 26911908.108. Kupfer ME, Lin WH, Ravikumar V, et al. In situ expansion, differentiation, and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ Res. 2020; 127:207–224. PMID: 32228120.109. Lee J, Sutani A, Kaneko R, et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat Commun. 2020; 11:4283. PMID: 32883967.110. Wu CC, Jeratsch S, Graumann J, Stainier DY. Modulation of mammalian cardiomyocyte cytokinesis by the extracellular matrix. Circ Res. 2020; 127:896–907. PMID: 32564729.111. Li Y, Asfour H, Bursac N. Age-dependent functional crosstalk between cardiac fibroblasts and cardiomyocytes in a 3D engineered cardiac tissue. Acta Biomater. 2017; 55:120–130. PMID: 28455218.112. Mastikhina O, Moon BU, Williams K, et al. Human cardiac fibrosis-on-a-chip model recapitulates disease hallmarks and can serve as a platform for drug testing. Biomaterials. 2020; 233:119741. PMID: 31927251.113. Sekine H, Shimizu T, Hobo K, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008; 118:S145–S152. PMID: 18824746.114. Choi A, Kim H, Han H, et al. Sutureless transplantation of in vivo priming human mesenchymal stem cell sheet promotes the therapeutic potential for cardiac repair. Biofabrication. 2023; 15:015009.115. Giacomelli E, Meraviglia V, Campostrini G, et al. Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell. 2020; 26:862–879.e11. PMID: 32459996.116. Lesman A, Habib M, Caspi O, et al. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010; 16:115–125. PMID: 19642856.117. Ishigami M, Masumoto H, Ikuno T, et al. Human iPS cell-derived cardiac tissue sheets for functional restoration of infarcted porcine hearts. PLoS One. 2018; 13:e0201650. PMID: 30071102.118. Huang K, Ozpinar EW, Su T, et al. An off-the-shelf artificial cardiac patch improves cardiac repair after myocardial infarction in rats and pigs. Sci Transl Med. 2020; 12:eaat9683. PMID: 32269164.119. Gaetani R, Doevendans PA, Metz CH, et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012; 33:1782–1790. PMID: 22136718.120. Gaetani R, Feyen DA, Verhage V, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015; 61:339–348. PMID: 26043062.121. Ong CS, Fukunishi T, Zhang H, et al. Biomaterial-free three-dimensional bioprinting of cardiac tissue using human induced pluripotent stem cell derived cardiomyocytes. Sci Rep. 2017; 7:4566. PMID: 28676704.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening of the Presence of Hepatitis B and C Virus Infections in Terminally Failing Human Hearts
- Troponin T and I Expression in Failing and Hypertrophic Heart, and during Normal Development in Human
- Three cases of multiple infarcted regenerative nodules in liver cirrhosis after gastrointestinal hemorrhage
- Development and Evaluation of a New Apparatus for Continuous Perfusion of Isolated Perfused Pig Heart
- Screening of the Presence of Enterovirus and Cytomegalovirus Infections in Terminally Failing Human Hearts