J Korean Med Sci.
2023 May;38(21):e167. 10.3346/jkms.2023.38.e167.
Cost-Effectiveness of All-Oral Regimens for the Treatment of Multidrug-Resistant Tuberculosis in Korea: Comparison With Conventional Injectable-Containing Regimens
- Affiliations
-
- 1BK21 FOUR Community-Based Intelligent Novel Drug Discovery Education Unit, College of Pharmacy and Research Institute of Pharmaceutical Sciences, Kyungpook National University, Daegu, Korea
- 2College of Pharmacy, Sahmyook University, Seoul, Korea
- 3School of Pharmacy, Sungkyunkwan University, Suwon, Korea
- 4Division of Big Data Science, Korea University Sejong Campus, Sejong, Korea
- 5Department of Biohealth Regulatory Science, Sungkyunkwan University, Suwon, Korea
- 6Department of Epidemiology, Biostatistics and Occupational Health, McGill University, Montréal, Québec, Canada
- 7Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2542602
- DOI: http://doi.org/10.3346/jkms.2023.38.e167
Abstract
- Background
Regimens for the treatment of multidrug-resistant tuberculosis (MDR-TB) have been changed from injectable-containing regimens to all-oral regimens. The economic effectiveness of new all-oral regimens compared with conventional injectable-containing regimens was scarcely evaluated. This study was conducted to compare the cost-effectiveness between all-oral longer-course regimens (the oral regimen group) and conventional injectablecontaining regimens (the control group) to treat newly diagnosed MDR-TB patients.
Methods
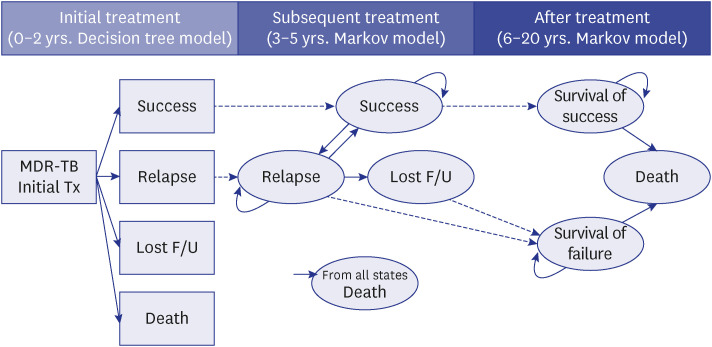
A health economic analysis over lifetime horizon (20 years) from the perspective of the healthcare system in Korea was conducted. We developed a combined simulation model of a decision tree model (initial two years) and two Markov models (remaining 18 years, sixmonth cycle length) to calculate the incremental cost-effectiveness ratio (ICER) between the two groups. The transition probabilities and cost in each cycle were assumed based on the published data and the analysis of health big data that combined country-level claims data and TB registry in 2013–2018.
Results
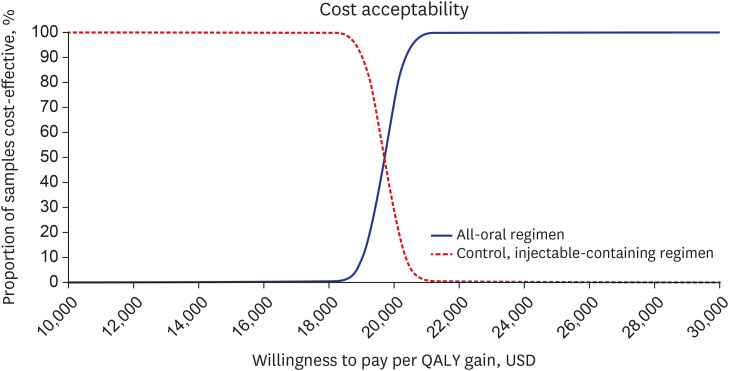
The oral regimen group was assumed to spend 20,778 USD more and lived 1.093 years or 1.056 quality-adjusted life year (QALY) longer than the control group. The ICER of the base case was calculated to be 19,007 USD/life year gained and 19,674 USD/QALY. The results of sensitivity analyses showed that base case results were very robust and stable, and the oral regimen was cost-effective with a 100% probability for a willingness to pay more than 21,250 USD/QALY.
Conclusion
This study confirmed that the new all-oral longer regimens for the treatment of MDR-TB were cost-effective in replacing conventional injectable-containing regimens.
Figure
Reference
-
1. World Health Organization. Global tuberculosis report 2021. Accessed in March 28, 2022. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globaltuberculosis-report-2021 .2. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: Treatment – drug-resistant tuberculosis treatment. Updated 2021. Accessed March 28, 2022. https://www.who.int/publications/i/item/9789240007048 .3. Korea Disease Control and Prevention Agency. Infectious disease portal, 2021. Tuberculosis notification annual report. Accessed March 28, 2022. https://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=1&pblctDtaSn=2333 .4. Cho KS. Tuberculosis control in the Republic of Korea. Epidemiol Health. 2018; 40:e2018036. PMID: 30081621.5. Kwak N, Hwang SS, Yim JJ. Effect of COVID-19 on tuberculosis notification, South Korea. Emerg Infect Dis. 2020; 26(10):2506–2508. PMID: 32672531.6. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017. Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JC, Anderson LF, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018; 392(10150):821–834. PMID: 30215381.7. Diacon AH, Pym A, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, et al. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014; 371(8):723–732. PMID: 25140958.8. Borisov SE, Dheda K, Enwerem M, Romero Leyet R, D’Ambrosio L, Centis R, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017; 49(5):1700387. PMID: 28529205.9. Guglielmetti L, Jaspard M, Le Dû D, Lachâtre M, Marigot-Outtandy D, Bernard C, et al. Long-term outcome and safety of prolonged bedaquiline treatment for multidrug-resistant tuberculosis. Eur Respir J. 2017; 49(3):1601799. PMID: 28182570.10. Tack I, Dumicho A, Ohler L, Shigayeva A, Bulti AB, White K, et al. Safety and effectiveness of an all-oral, bedaquiline-based, shorter treatment regimen for rifampicin-resistant tuberculosis in high human immunodeficiency virus (HIV) burden rural South Africa: a retrospective cohort analysis. Clin Infect Dis. 2021; 73(9):e3563–e3571. PMID: 33372989.11. Padayatchi N, Bionghi N, Osman F, Naidu N, Ndjeka N, Master I, et al. Treatment outcomes in patients with drug-resistant TB-HIV co-infection treated with bedaquiline and linezolid. Int J Tuberc Lung Dis. 2020; 24(10):1024–1031. PMID: 33126934.12. Park HY, Ku HM, Sohn HS, Seo HS, Yung Lee H, Hwa Lim K, et al. Cost-effectiveness of bedaquiline for the treatment of multidrug-resistant tuberculosis in the Republic of Korea. Clin Ther. 2016; 38(3):655–667.e1-2. PMID: 26907504.13. Mpobela Agnarson A, Williams A, Kambili C, Mattson G, Metz L. The cost-effectiveness of a bedaquiline-containing short-course regimen for the treatment of multidrug-resistant tuberculosis in South Africa. Expert Rev Anti Infect Ther. 2020; 18(5):475–483. PMID: 32186925.14. Ionescu AM, Mpobela Agnarson A, Kambili C, Metz L, Kfoury J, Wang S, et al. Bedaquiline- versus injectable-containing drug-resistant tuberculosis regimens: a cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. 2018; 18(6):677–689. PMID: 30073886.15. Gomez GB, Siapka M, Conradie F, Ndjeka N, Garfin AM, Lomtadze N, et al. Cost-effectiveness of bedaquiline, pretomanid and linezolid for treatment of extensively drug-resistant tuberculosis in South Africa, Georgia and the Philippines. BMJ Open. 2021; 11(12):e051521.16. The Korean Academy of Tuberculosis and Respiratory Disease. Korea Disease Control and Prevention Agency. Guideline of Tuberculosis Management. 4th ed. Cheongju, Korea: Korea Disease Control and Prevention Agency;2020.17. Kittikraisak W, Kingkaew P, Teerawattananon Y, Yothasamut J, Natesuwan S, Manosuthi W, et al. Health related quality of life among patients with tuberculosis and HIV in Thailand. PLoS One. 2012; 7(1):e29775. PMID: 22253777.18. Agnarson AM, Wang XC, Potluri R, Bhandari H, Dhir A, Kambili C, et al. Long-term impact of the adoption of bedaquiline-containing regimens on the burden of drug-resistant tuberculosis in China. BMC Infect Dis. 2020; 20(1):113. PMID: 32041542.19. Lee M, Han J, Kim YR, Kwak N, Kim JH, Park O, et al. Multidrug-resistant tuberculosis in South Korea: a retrospective analysis of national registry data in 2011-2015. Int J Tuberc Lung Dis. 2019; 23(7):850–857. PMID: 31439118.20. World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision: updated December 2014 and January 2020. Accessed June 1, 2022. https://apps.who.int/iris/handle/10665/79199 .21. Hwang H, Kang H, Kwon YS, Jeon D, Shim TS, Yim JJ. Outcomes of multidrug-resistant tuberculosis treated with bedaquiline or delamanid. Clin Infect Dis. 2021; 73(8):1362–1369. PMID: 33837767.22. Lee HH, Jo KW, Yim JJ, Jeon D, Kang H, Shim TS. Interim treatment outcomes in multidrug-resistant tuberculosis patients treated sequentially with bedaquiline and delamanid. Int J Infect Dis. 2020; 98:478–485. PMID: 32640367.23. Alipanah N, Jarlsberg L, Miller C, Linh NN, Falzon D, Jaramillo E, et al. Adherence interventions and outcomes of tuberculosis treatment: a systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018; 15(7):e1002595. PMID: 29969463.24. Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One. 2011; 6(4):e17601. PMID: 21483732.25. Masuku SD, Berhanu R, Van Rensburg C, Ndjeka N, Rosen S, Long L, et al. Managing multidrug-resistant tuberculosis in South Africa: a budget impact analysis. Int J Tuberc Lung Dis. 2020; 24(4):376–382. PMID: 32317060.26. Kwon YS, Jeon D, Kang H, Yim JJ, Shim TS. Concurrent use of bedaquiline and delamanid for the treatment of fluoroquinolone-resistant multidrug-resistant tuberculosis: a nationwide cohort study in South Korea. Eur Respir J. 2021; 57(3):2003026. PMID: 33093123.27. Park HY, Cheon HB, Choi SH, Kwon JW. Health-related quality of life based on EQ-5D utility score in patients with tuberculosis: a systematic review. Front Pharmacol. 2021; 12:659675. PMID: 33935781.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Shorter Oral Regimen for Multidrug Resistant Tuberculosis in South Korea
- Clinical Implications of New Drugs and Regimens for the Treatment of Drug-resistant Tuberculosis
- Multidrug-resistant Tuberculosis
- WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update: Applicability in South Korea
- Medical Management of Drug-Resistant Tuberculosis