Neonatal Med.
2023 May;30(2):34-41. 10.5385/nm.2023.30.2.34.
Effects of Recombinant Human Erythropoietin Administration in Premature Infants with Severe Intraventricular Hemorrhage: A Single-Center Experience
- Affiliations
-
- 1Department of Pediatrics, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine Seoul, Korea
- 2Department of Pediatrics, Kyung Hee University Hospital at Gangdong, Seoul, Korea
- KMID: 2542545
- DOI: http://doi.org/10.5385/nm.2023.30.2.34
Abstract
- Purpose
We investigated the effects of early postnatal administration of erythropoietin (EPO) on neurodevelopmental outcomes and morbidities in preterm infants with severe grades of intraventricular hemorrhage (IVH).
Methods
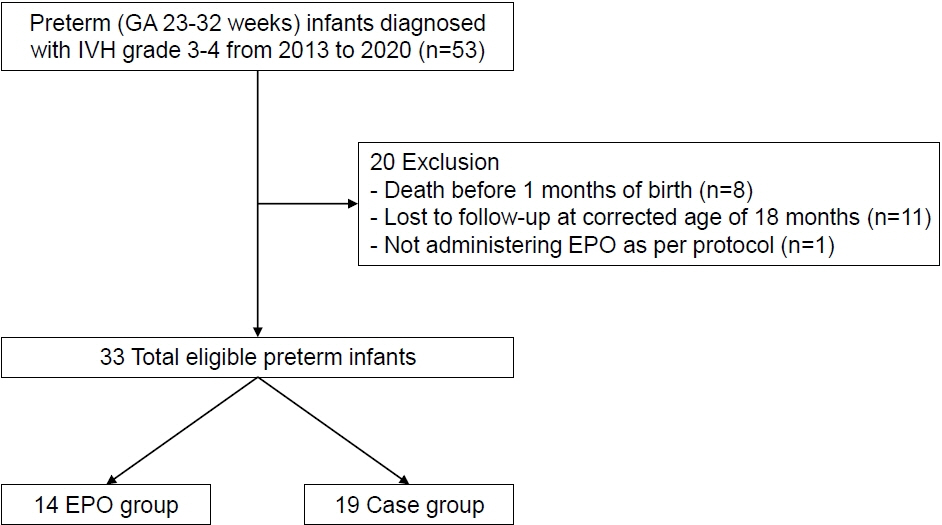
We retrospectively reviewed the medical records of preterm infants of gestational age 23+0 weeks to 31+6 weeks, who were diagnosed with severe grades of IVH and received EPO over at least 2 weeks. We compared clinical characteristics, major complications, and neurodevelopmental impairment between the two groups. The primary outcome was severe neurodevelopmental impairment at 18 to 26 months of corrected age. Severe neurodevelopmental impairment was defined as a mental developmental index or psychomotor developmental index of <70 on the Bayley Scales of Infant Development II or diagnosis of cerebral palsy.
Results
The study included 33 preterm infants (mean gestational age 25.2±1.6 weeks and mean birth weight 775.1±224.5 g). EPO was administered at a dose of 400 or 1,000 IU/kg thrice weekly and was maintained over a mean period of 58.6± 25.9 days beginning from 10.7±6.9 days after birth. We observed no difference in perinatal characteristics between the EPO (n=14) and the control group (n=19). Similarly, severe neurodevelopmental impairment rates did not differ between the EPO (85.7%) and control groups (78.9%). The incidence of neonatal morbidities including bronchopulmonary dysplasia, necrotizing enterocolitis, and retinopathy of prematurity was also similar between the EPO and control groups.
Conclusion
Early administration of EPO did not reduce the risk of severe neurodevelopmental impairment in preterm infants with severe IVH.
Figure
Reference
-
1. Jain NJ, Kruse LK, Demissie K, Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. J Matern Fetal Neonatal Med. 2009; 22:491–500.
Article2. Singhi S, Johnston M. Recent advances in perinatal neuroprotection. F1000Res. 2019; 8(F1000 Faculty Rev):2031.
Article3. McNally MA, Soul JS. Pharmacologic prevention and treatment of neonatal brain injury. Clin Perinatol. 2019; 46:311–25.
Article4. Victor S, Rocha-Ferreira E, Rahim A, Hagberg H, Edwards D. New possibilities for neuroprotection in neonatal hypoxicischemic encephalopathy. Eur J Pediatr. 2022; 181:875–87.
Article5. Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007; 64:159–71.
Article6. Kumral A, Tuzun F, Oner MG, Genc S, Duman N, Ozkan H. Erythropoietin in neonatal brain protection: the past, the present and the future. Brain Dev. 2011; 33:632–43.
Article7. Brown MS, Eichorst D, Lala-Black B, Gonzalez R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2009; 124:e681–7.
Article8. Fischer HS, Reibel NJ, Buhrer C, Dame C. Prophylactic early erythropoietin for neuroprotection in preterm infants: a metaanalysis. Pediatrics. 2017; 139:e20164317.
Article9. Song J, Wang Y, Xu F, Sun H, Zhang X, Xia L, et al. Erythropoietin improves poor outcomes in preterm infants with intraventricular hemorrhage. CNS Drugs. 2021; 35:681–90.
Article10. Wu YW, Bauer LA, Ballard RA, Ferriero DM, Glidden DV, Mayock DE, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics. 2012; 130:683–91.
Article11. Ohls RK, Kamath-Rayne BD, Christensen RD, Wiedmeier SE, Rosenberg A, Fuller J, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014; 133:1023–30.
Article12. Natalucci G, Latal B, Koller B, Ruegger C, Sick B, Held L, et al. Effect of early prophylactic high-dose recombinant human erythropoietin in very preterm infants on neurodevelopmental outcome at 2 years: a randomized clinical trial. JAMA. 2016; 315:2079–85.
Article13. Song J, Sun H, Xu F, Kang W, Gao L, Guo J, et al. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann Neurol. 2016; 80:24–34.
Article14. Ohls RK, Ehrenkranz RA, Das A, Dusick AM, Yolton K, Romano E, et al. Neurodevelopmental outcome and growth at 18 to 22 months’ corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics. 2004; 114:1287–91.
Article15. Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006; 118:e635–40.
Article16. Juul SE, Comstock BA, Wadhawan R, Mayock DE, Courtney SE, Robinson T, et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N Engl J Med. 2020; 382:233–43.
Article17. Kellert BA, McPherson RJ, Juul SE. A comparison of high-dose recombinant erythropoietin treatment regimens in braininjured neonatal rats. Pediatr Res. 2007; 61:451–5.
Article18. Gumy-Pause F, Ozsahin H, Mermillod B, Cingria L, Berner M, Wacker P. Stepping up versus standard doses of erythropoietin in preterm infants: a randomized controlled trial. Pediatr Hematol Oncol. 2005; 22:667–78.
Article19. Whitelaw A, Evans D, Carter M, Thoresen M, Wroblewska J, Mandera M, et al. Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics. 2007; 119:e1071–8.
Article20. Luyt K, Jary SL, Lea CL, Young GJ, Odd DE, Miller HE, et al. Drainage, irrigation and fibrinolytic therapy (DRIFT) for posthaemorrhagic ventricular dilatation: 10-year follow-up of a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2020; 105:466–73.
Article21. Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010; 67:657–66.
Article22. Aher SM, Ohlsson A. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2012; 10:CD004865.
Article23. Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008; 118:526–33.
Article24. Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005; 353:782–92.
Article25. Schmidt B, Davis PG, Asztalos EV, Solimano A, Roberts RS. Association between severe retinopathy of prematurity and nonvisual disabilities at age 5 years. JAMA. 2014; 311:523–5.
Article26. Ahn JH, Lee KM, Kim MJ, Park HK, Kim YJ, Ahn SJ, et al. Neurodevelopmental outcomes in very low birthweight infants with retinopathy of prematurity in a nationwide cohort study. Sci Rep. 2022; 12:5053.
Article27. Jensen EA, Edwards EM, Greenberg LT, Soll RF, Ehret DE, Horbar JD. Severity of bronchopulmonary dysplasia among very preterm infants in the United States. Pediatrics. 2021; 148:e2020030007.
Article28. Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol. 2006; 30:227–32.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Prophylactic Treatment of High Doses Recombinant Human Erythropoietin on Anemia in Premature Infants
- Pharmacological Management of Germinal Matrix-Intraventricular Hemorrhage
- Clinical Trials for Preterm Infants' Neurodevelopment to the Norm: Erythropoietin and Nutritional Interventions
- Effect of Recombinant Human Erythropoietin in the Anemia of Prematurity : a Pilot Study
- Neuroimaging of Germinal Matrix and Intraventricular Hemorrhage in Premature Infants