Diabetes Metab J.
2023 May;47(3):366-381. 10.4093/dmj.2022.0179.
Hyperglycemia-Suppressed SMARCA5 Disrupts Transcriptional Homeostasis to Facilitate Endothelial Dysfunction in Diabetes
- Affiliations
-
- 1Departments of Nursing, Xi’an No. 5 Hospital, Xi’an, China
- 2Departments of Geriatric, Xi’an No. 5 Hospital, Xi’an, China
- KMID: 2542514
- DOI: http://doi.org/10.4093/dmj.2022.0179
Abstract
- Background
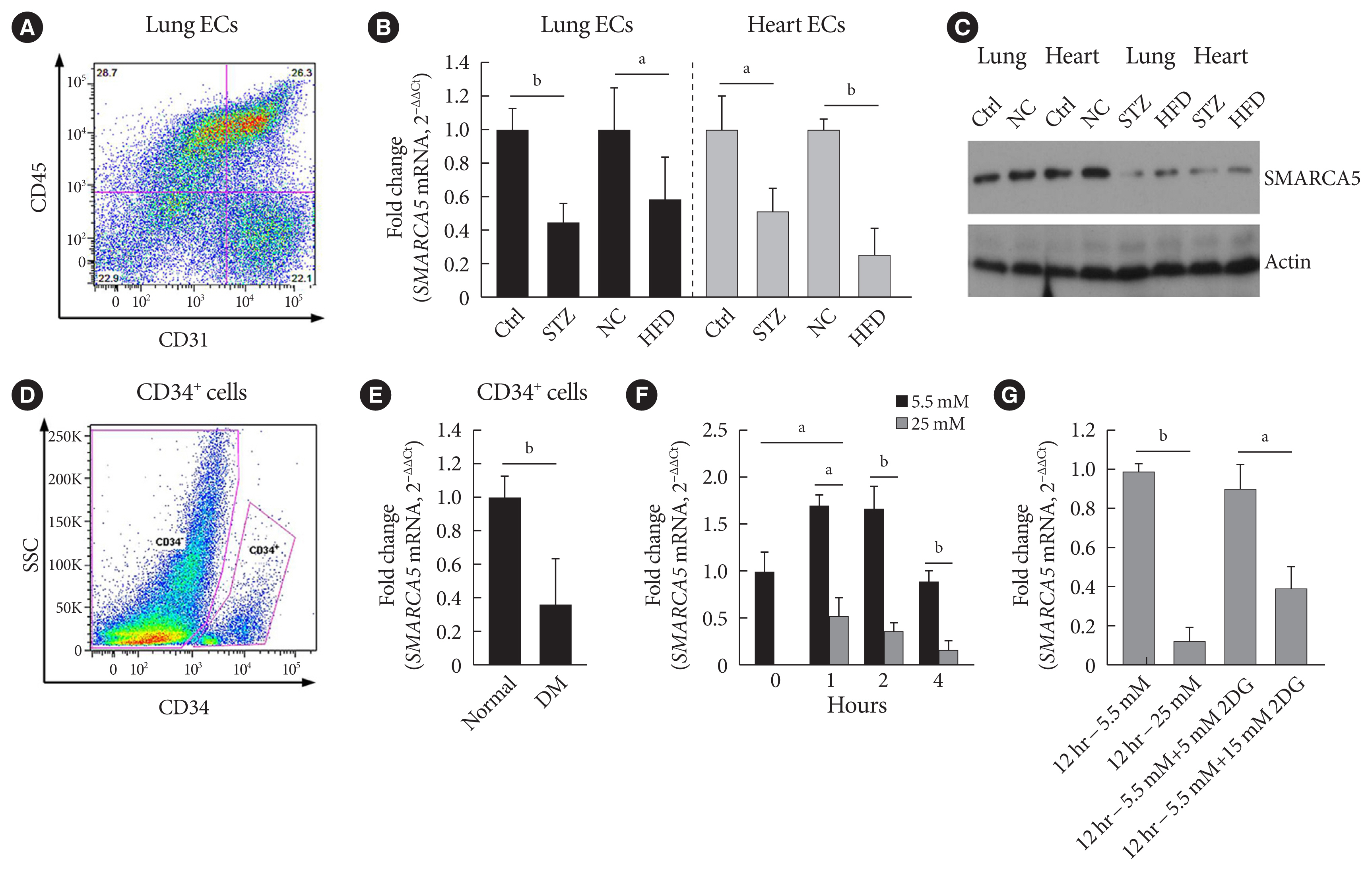
Dysfunction of vascular endothelial cells (ECs) plays a central role in the pathogenesis of cardiovascular complications in diabetes. SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily A member 5 (SMARCA5) is a key regulator of chromatin structure and DNA repair, but its role in ECs remains surprisingly unexplored. The current study was designed to elucidate the regulated expression and function of SMARCA5 in diabetic ECs.
Methods
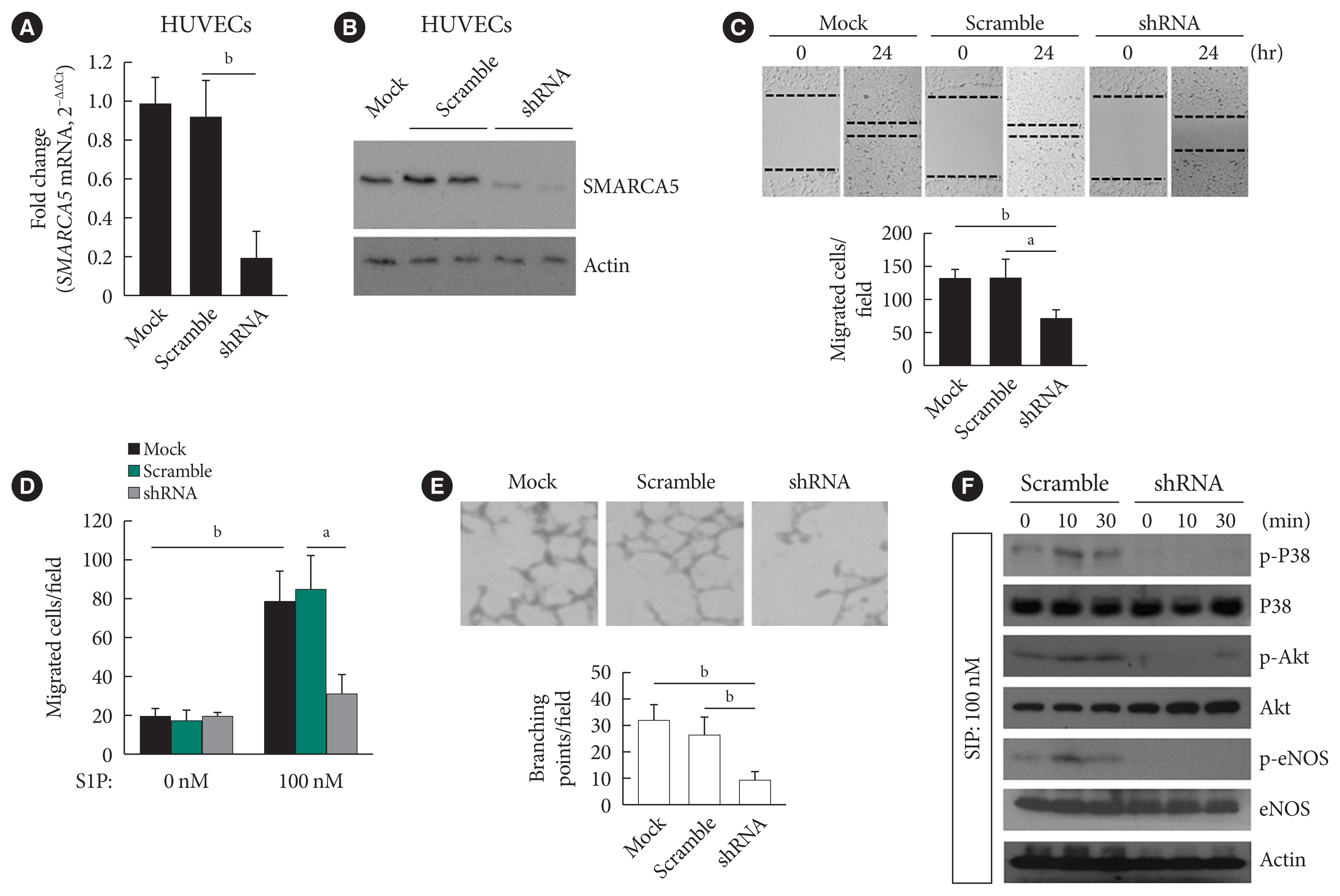
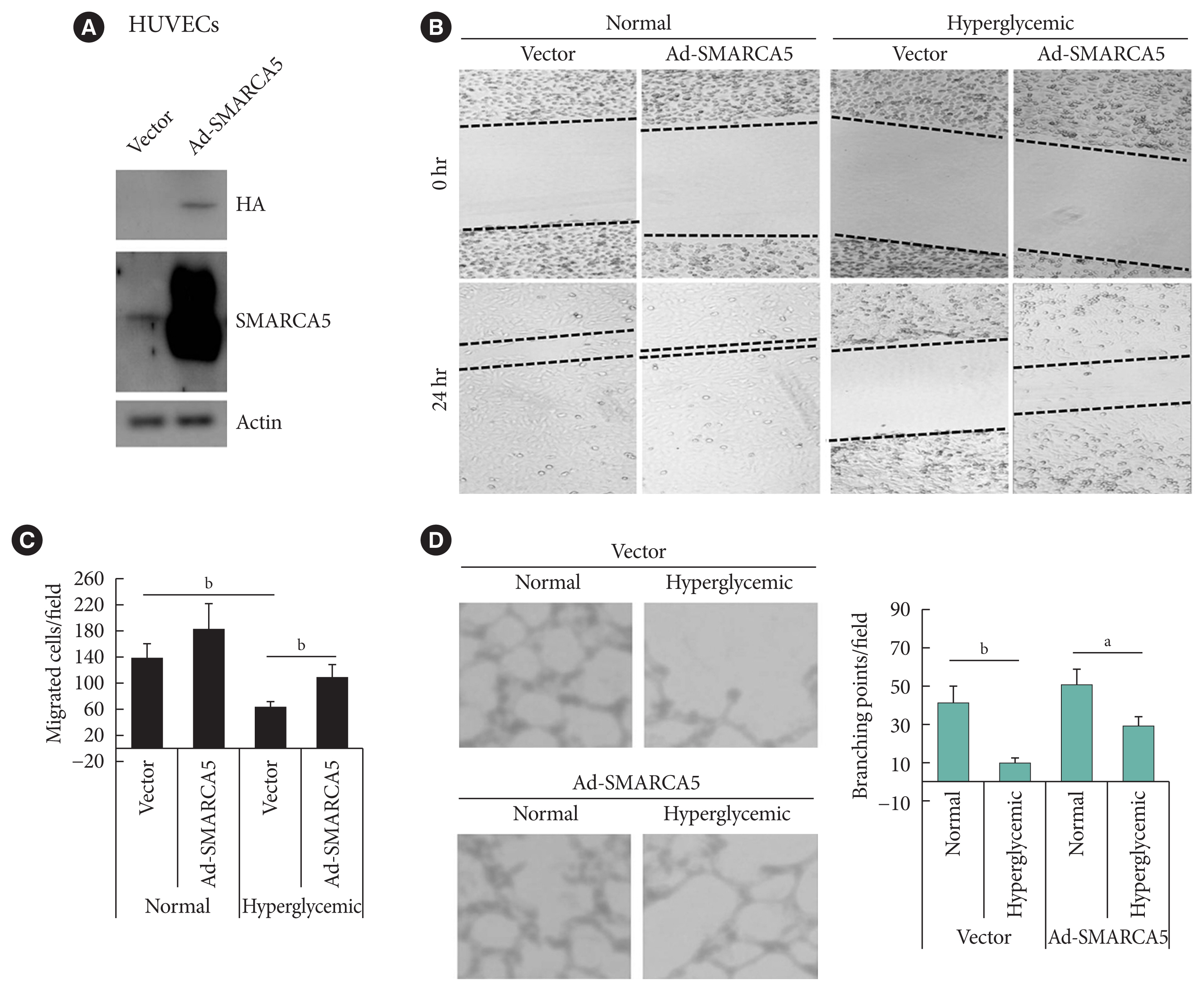
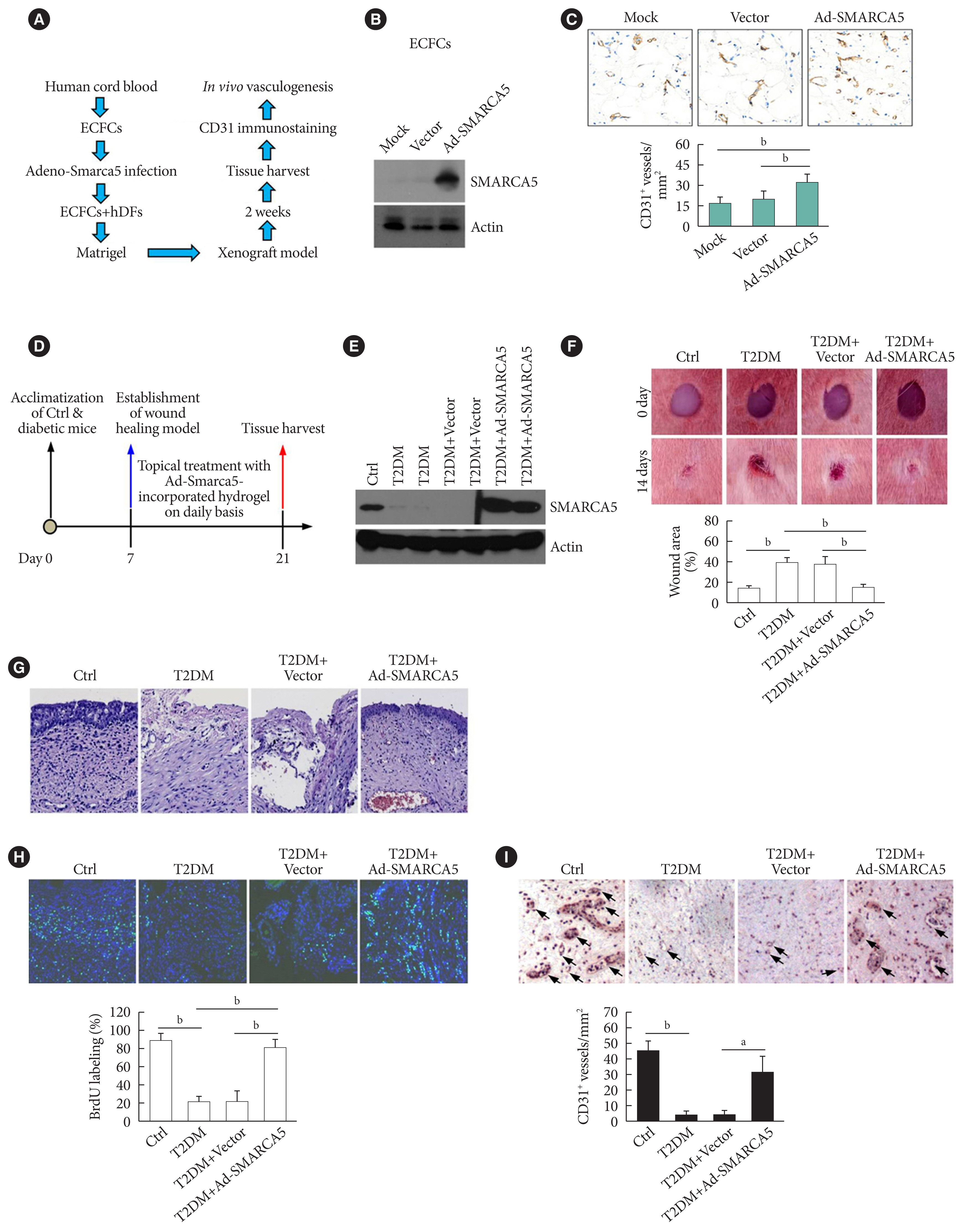
SMARCA5 expression was evaluated in ECs from diabetic mouse and human circulating CD34+ cells using quantitative reverse transcription polymerase chain reaction and Western blot. Effects of SMARCA5 manipulation on ECs function were evaluated using cell migration, in vitro tube formation and in vivo wound healing assays. Interaction among oxidative stress, SMARCA5 and transcriptional reprogramming was elucidated using luciferase reporter assay, electrophoretic mobility shift assay and chromatin immunoprecipitation.
Results
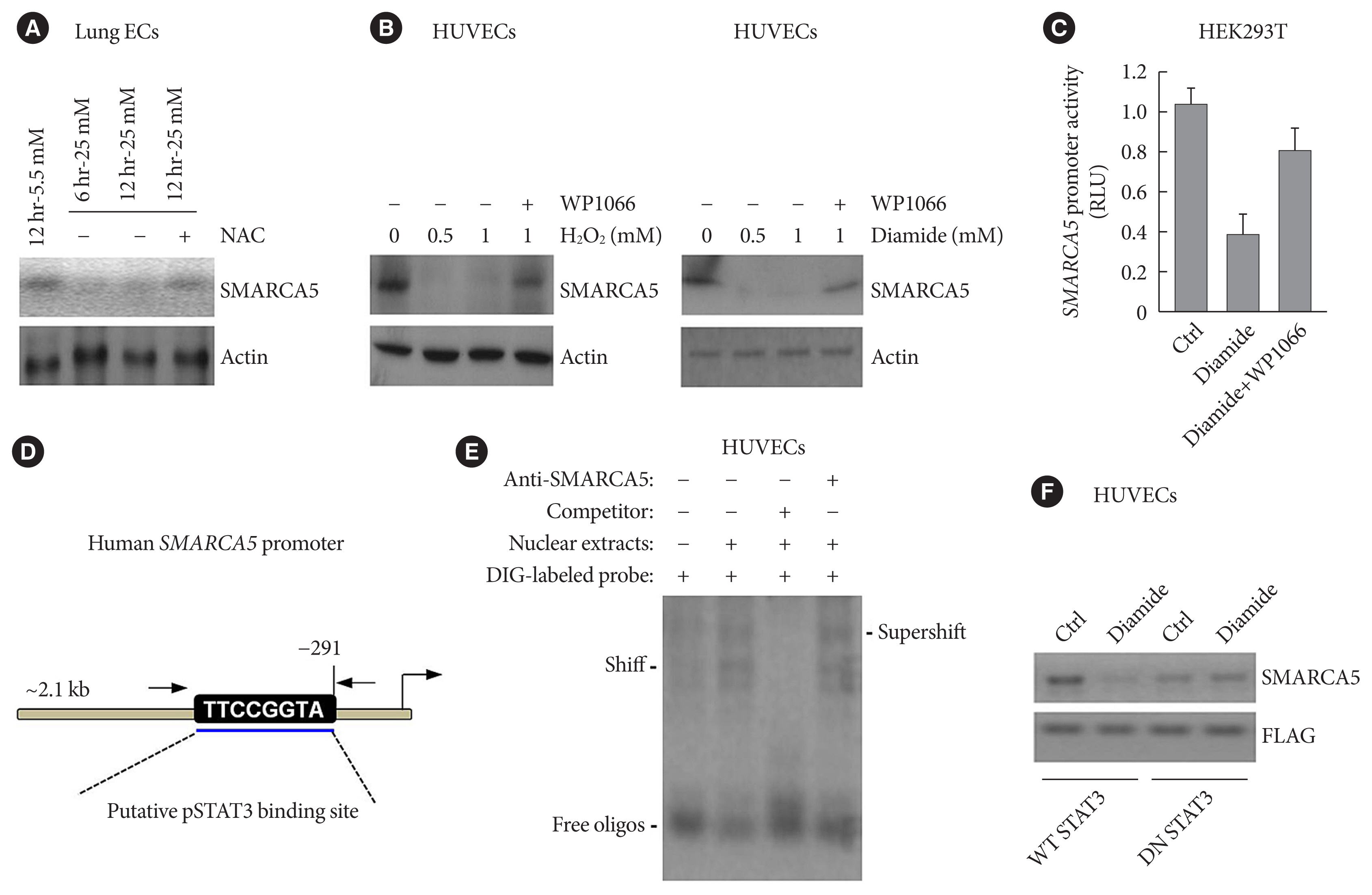
Endothelial SMARCA5 expression was significantly decreased in diabetic rodents and humans. Hyperglycemia-suppressed SMARCA5 impaired EC migration and tube formation in vitro, and blunted vasculogenesis in vivo. Contrarily, overexpression of SMARCA5 in situ by a SMARCA5 adenovirus-incorporated hydrogel effectively promoted the rate of wound healing in a dorsal skin punch injury model of diabetic mice. Mechanistically, hyperglycemia-elicited oxidative stress suppressed SMARCA5 transactivation in a signal transducer and activator of transcription 3 (STAT3)-dependent manner. Moreover, SMARCA5 maintained transcriptional homeostasis of several pro-angiogenic factors through both direct and indirect chromatin-remodeling mechanisms. In contrast, depletion of SMARCA5 disrupted transcriptional homeostasis to render ECs unresponsive to established angiogenic factors, which ultimately resulted in endothelial dysfunction in diabetes.
Conclusion
Suppression of endothelial SMARCA5 contributes to, at least in part, multiple aspects of endothelial dysfunction, which may thereby exacerbate cardiovascular complications in diabetes.
Keyword
Figure
Reference
-
1. Knapp M, Tu X, Wu R. Vascular endothelial dysfunction, a major mediator in diabetic cardiomyopathy. Acta Pharmacol Sin. 2019; 40:1–8.2. Yang C, Eleftheriadou M, Kelaini S, Morrison T, Gonzalez MV, Caines R, et al. Targeting QKI-7 in vivo restores endothelial cell function in diabetes. Nat Commun. 2020; 11:3812.3. Aiello LP, Wong JS. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int Suppl. 2000; 77:S113–9.4. Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2012; 2012:918267.
Article5. Menon DU, Kirsanov O, Geyer CB, Magnuson T. Mammalian SWI/SNF chromatin remodeler is essential for reductional meiosis in males. Nat Commun. 2021; 12:6581.6. Thakur S, Cahais V, Turkova T, Zikmund T, Renard C, Stopka T, et al. Chromatin remodeler smarca5 is required for cancer-related processes of primary cell fitness and immortalization. Cells. 2022; 11:808.7. Jevtic Z, Matafora V, Casagrande F, Santoro F, Minucci S, Garre M, et al. SMARCA5 interacts with NUP98-NSD1 oncofusion protein and sustains hematopoietic cells transformation. J Exp Clin Cancer Res. 2022; 41:34.8. Zikmund T, Paszekova H, Kokavec J, Kerbs P, Thakur S, Turkova T, et al. Loss of ISWI ATPase SMARCA5 (SNF2H) in acute myeloid leukemia cells inhibits proliferation and chromatid cohesion. Int J Mol Sci. 2020; 21:2073.9. Cui T, Bell EH, McElroy J, Liu K, Sebastian E, Johnson B, et al. A novel miR-146a-POU3F2/SMARCA5 pathway regulates stemness and therapeutic response in glioblastoma. Mol Cancer Res. 2021; 19:48–60.
Article10. Jin Q, Mao X, Li B, Guan S, Yao F, Jin F. Overexpression of SMARCA5 correlates with cell proliferation and migration in breast cancer. Tumour Biol. 2015; 36:1895–902.11. Ai C, Ma G, Deng Y, Zheng Q, Gen Y, Li W, et al. Nm23-H1 inhibits lung cancer bone-specific metastasis by upregulating miR-660-5p targeted SMARCA5. Thorac Cancer. 2020; 11:640–50.12. Gigek CO, Lisboa LC, Leal MF, Silva PN, Lima EM, Khayat AS, et al. SMARCA5 methylation and expression in gastric cancer. Cancer Invest. 2011; 29:162–6.13. Ding Y, Li Y, Zhao Z, Cliff Zhang Q, Liu F. The chromatin-remodeling enzyme Smarca5 regulates erythrocyte aggregation via Keap1-Nrf2 signaling. Elife. 2021; 10:e72557.14. Ding Y, Wang W, Ma D, Liang G, Kang Z, Xue Y, et al. Smarca5-mediated epigenetic programming facilitates fetal HSPC development in vertebrates. Blood. 2021; 137:190–202.
Article15. Dong YS, Hou WG, Li Y, Liu DB, Hao GZ, Zhang HF, et al. Unexpected requirement for a binding partner of the syntaxin family in phagocytosis by murine testicular Sertoli cells. Cell Death Differ. 2016; 23:787–800.16. Chai X, Yan J, Gao Y, Jin J. Endothelial HNF4α potentiates angiogenic dysfunction via enhancement of vascular endothelial growth factor resistance in T2DM. J Cell Biochem. 2019; 120:12989–3000.17. Hao JS, Zhu CJ, Yan BY, Yan CY, Ling R. Stimulation of KLF14/PLK1 pathway by thrombin signaling potentiates endothelial dysfunction in type 2 diabetes mellitus. Biomed Pharmacother. 2018; 99:859–66.18. He K, Qu H, Wang H, Zhang S, Qian XH, Li W. Regulated and functional expression of the corepressor MTA3 in rodent testis. Endocrinology. 2016; 157:4400–10.19. Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, et al. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006; 26:2140–6.20. Sawada N, Jiang A, Takizawa F, Safdar A, Manika A, Tesmenitsky Y, et al. Endothelial PGC-1α mediates vascular dysfunction in diabetes. Cell Metab. 2014; 19:246–58.21. Cheng S, Cui Y, Fan L, Mu X, Hua Y. T2DM inhibition of endothelial miR-342-3p facilitates angiogenic dysfunction via repression of FGF11 signaling. Biochem Biophys Res Commun. 2018; 503:71–8.22. Niccoli S, Boreham DR, Phenix CP, Lees SJ. Non-radioactive 2-deoxy-2-fluoro-D-glucose inhibits glucose uptake in xenograft tumours and sensitizes HeLa cells to doxorubicin in vitro. PLoS One. 2017; 12:e0187584.23. Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, et al. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007; 56:1559–68.24. Qi X, Okamoto Y, Murakawa T, Wang F, Oyama O, Ohkawa R, et al. Sustained delivery of sphingosine-1-phosphate using poly(lactic-co-glycolic acid)-based microparticles stimulates Akt/ERK-eNOS mediated angiogenesis and vascular maturation restoring blood flow in ischemic limbs of mice. Eur J Pharmacol. 2010; 634:121–31.25. Tie L, Li XJ, Wang X, Channon KM, Chen AF. Endothelium-specific GTP cyclohydrolase I overexpression accelerates refractory wound healing by suppressing oxidative stress in diabetes. Am J Physiol Endocrinol Metab. 2009; 296:E1423–9.26. Marshall CD, Hu MS, Leavitt T, Barnes LA, Cheung AT, Malhotra S, et al. Sanativo wound healing product does not accelerate reepithelialization in a mouse cutaneous wound healing model. Plast Reconstr Surg. 2017; 139:343–52.27. Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013; 19:5695–703.28. Ning P, Zhong JG, Jiang F, Zhang Y, Zhao J, Tian F, et al. Role of protein S in castration-resistant prostate cancer-like cells. Endocr Relat Cancer. 2016; 23:595–607.29. Jin X, Zhang S, Ding T, Zhao P, Zhang C, Zhang Y, et al. Testicular Lmcd1 regulates phagocytosis by Sertoli cells through modulation of NFAT1/Txlna signaling pathway. Aging Cell. 2020; 19:e13217.30. Wang GG, Wang YZ, Xie J, Huang CY, Kong ZL, Ding X, et al. Cyclic tensile forces enhance the angiogenic properties of HUVECs by promoting the activities of human periodontal ligament cells. J Periodontol. 2021; 92:159–69.31. Luo Q, Wu X, Chang W, Zhao P, Zhu X, Chen H, et al. ARID1A hypermethylation disrupts transcriptional homeostasis to promote squamous cell carcinoma progression. Cancer Res. 2020; 80:406–17.32. Liu J, Li C, Wang J, Xu D, Wang H, Wang T, et al. Chromatin modifier MTA1 regulates mitotic transition and tumorigenesis by orchestrating mitotic mRNA processing. Nat Commun. 2020; 11:4455.33. Monteiro JP, Bennett M, Rodor J, Caudrillier A, Ulitsky I, Baker AH. Endothelial function and dysfunction in the cardiovascular system: the long non-coding road. Cardiovasc Res. 2019; 115:1692–704.34. Kumar S. SWI/SNF (BAF) complexes: from framework to a functional role in endothelial mechanotransduction. Curr Top Membr. 2021; 87:171–98.35. Griffin CT, Brennan J, Magnuson T. The chromatin-remodeling enzyme BRG1 plays an essential role in primitive erythropoiesis and vascular development. Development. 2008; 135:493–500.36. Huang X, Gao X, Diaz-Trelles R, Ruiz-Lozano P, Wang Z. Coronary development is regulated by ATP-dependent SWI/SNF chromatin remodeling component BAF180. Dev Biol. 2008; 319:258–66.37. Moonen JR, Chappell J, Shi M, Shinohara T, Li D, Mumbach MR, et al. KLF4 recruits SWI/SNF to increase chromatin accessibility and reprogram the endothelial enhancer landscape under laminar shear stress. Nat Commun. 2022; 13:4941.38. Ishikura K, Misu H, Kumazaki M, Takayama H, Matsuzawa-Nagata N, Tajima N, et al. Selenoprotein P as a diabetes-associated hepatokine that impairs angiogenesis by inducing VEGF resistance in vascular endothelial cells. Diabetologia. 2014; 57:1968–76.39. Alhusban A, Alkhazaleh E, El-Elimat T. Silymarin ameliorates diabetes-induced proangiogenic response in brain endothelial cells through a GSK-3β inhibition-induced reduction of VEGF release. J Diabetes Res. 2017; 2017:2537216.40. Xia L, Huang W, Bellani M, Seidman MM, Wu K, Fan D, et al. CHD4 has oncogenic functions in initiating and maintaining epigenetic suppression of multiple tumor suppressor genes. Cancer Cell. 2017; 31:653–68e7.41. McKenzie LD, LeClair JW, Miller KN, Strong AD, Chan HL, Oates EL, et al. CHD4 regulates the DNA damage response and RAD51 expression in glioblastoma. Sci Rep. 2019; 9:4444.42. Yun JH, Park SW, Kim KJ, Bae JS, Lee EH, Paek SH, et al. Endothelial STAT3 activation increases vascular leakage through downregulating tight junction proteins: implications for diabetic retinopathy. J Cell Physiol. 2017; 232:1123–34.43. Levings DC, Lacher SE, Palacios-Moreno J, Slattery M. Transcriptional reprogramming by oxidative stress occurs within a predefined chromatin accessibility landscape. Free Radic Biol Med. 2021; 171:319–31.44. Tommasi S, Pinto R, Danza K, Pilato B, Palumbo O, Micale L, et al. miR-151-5p, targeting chromatin remodeler SMARCA5, as a marker for the BRCAness phenotype. Oncotarget. 2016; 7:80363–72.45. Wu Z, Tang H, Xiong Q, Liu D, Xia T, Liang H, et al. Prognostic role of microRNA-205 in human gynecological cancer: a meta-analysis of fourteen studies. DNA Cell Biol. 2020; 39:875–89.46. Ouni M, Gottmann P, Westholm E, Schwerbel K, Jahnert M, Stadion M, et al. MiR-205 is up-regulated in islets of diabetes-susceptible mice and targets the diabetes gene Tcf7l2. Acta Physiol (Oxf). 2021; 232:e13693.47. Zhang Q, Xiao X, Li M, Li W, Yu M, Zhang H, et al. Acarbose reduces blood glucose by activating miR-10a-5p and miR-664 in diabetic rats. PLoS One. 2013; 8:e79697.48. Sugden WW, North TE. Making blood from the vessel: extrinsic and environmental cues guiding the endothelial-to-hematopoietic transition. Life (Basel). 2021; 11:1027.49. Kokavec J, Zikmund T, Savvulidi F, Kulvait V, Edelmann W, Skoultchi AI, et al. The ISWI ATPase Smarca5 (Snf2h) is required for proliferation and differentiation of hematopoietic stem and progenitor cells. Stem Cells. 2017; 35:1614–23.50. Norton KA, Popel AS. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci Rep. 2016; 6:36992.51. Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005; 69(Suppl 3):4–10.52. Oyama Y, Shigeta S, Tokunaga H, Tsuji K, Ishibashi M, Shibuya Y, et al. CHD4 regulates platinum sensitivity through MDR1 expression in ovarian cancer: a potential role of CHD4 inhibition as a combination therapy with platinum agents. PLoS One. 2021; 16:e0251079.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Acute Hyperglycemia on Endothelial Function in Type 2 Diabetes
- Hyperglycemia-induced Activation of Nuclear Transcription Factor kappaB in Cultured Fibroblasts and Endothelial Cells
- Effects of Acute Hyperglycemia on Endothelium-Dependent Vasodilation in Patients with Diabetes Mellitus or Impaired Glucose Metabolism
- Diabetes Mellitus and Endothelial Cell Dysfunction
- Effect of Diabetes in Surgery