Diabetes Metab J.
2023 May;47(3):356-365. 10.4093/dmj.2022.0129.
Safety of COVID-19 Vaccines among Patients with Type 2 Diabetes Mellitus: Real-World Data Analysis
- Affiliations
-
- 1Department of Biomedical Informatics, CHA University School of Medicine, Seongnam, Korea
- 2Institute of Basic Medical Sciences, CHA University School of Medicine, Seongnam, Korea
- 3Institute for Biomedical Informatics, CHA University School of Medicine, Seongnam, Korea
- 4Department of Internal Medicine and Healthcare Research Institute, Seoul National University Hospital Healthcare System Gangnam Center, Seoul, Korea
- 5Department of Health and Medical Informatics, Kyungnam University College of Health Sciences, Changwon, Korea
- KMID: 2542513
- DOI: http://doi.org/10.4093/dmj.2022.0129
Abstract
- Background
Little is known about the adverse events (AEs) associated with coronavirus disease 2019 (COVID-19) vaccination in patients with type 2 diabetes mellitus (T2DM).
Methods
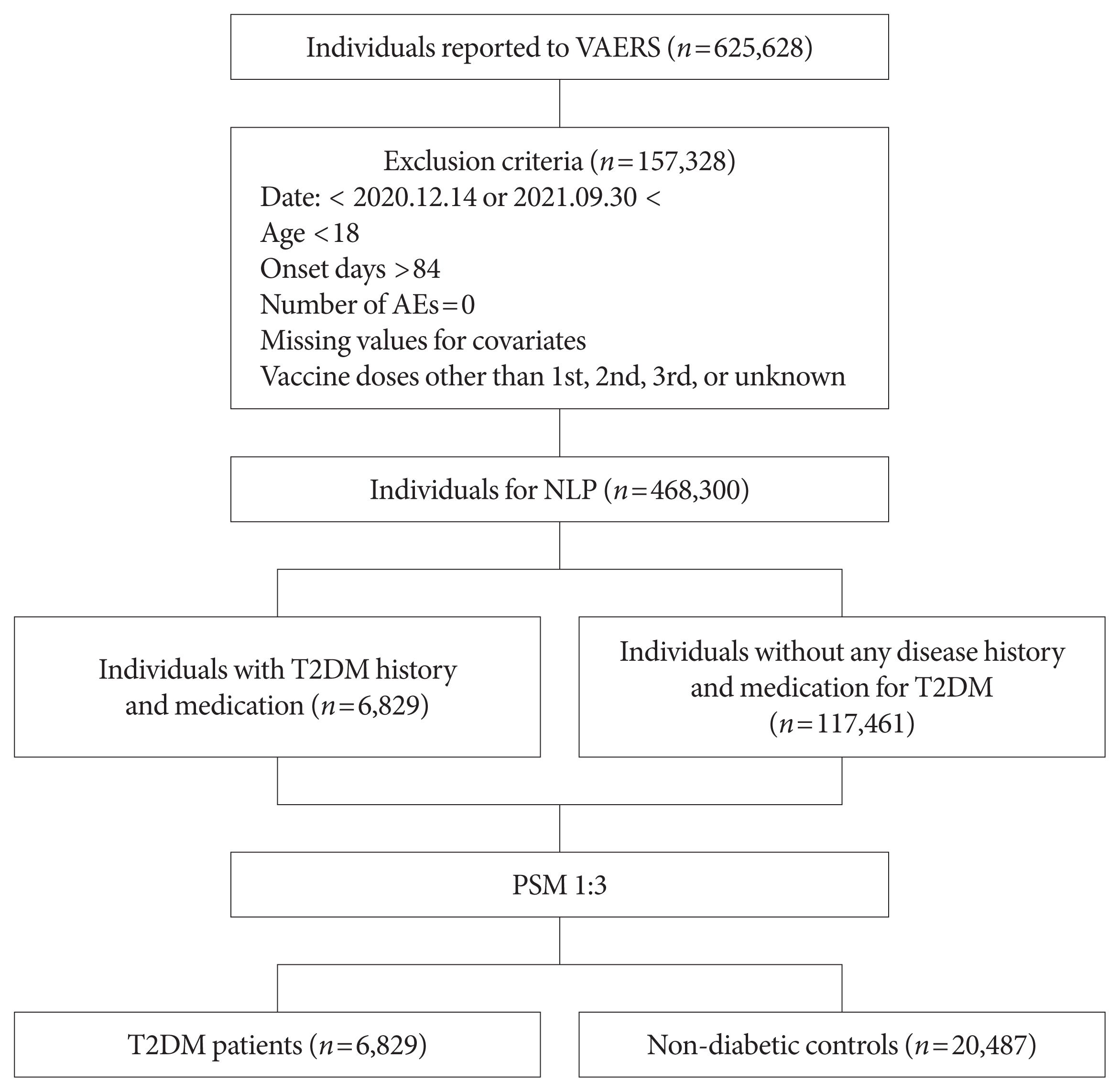
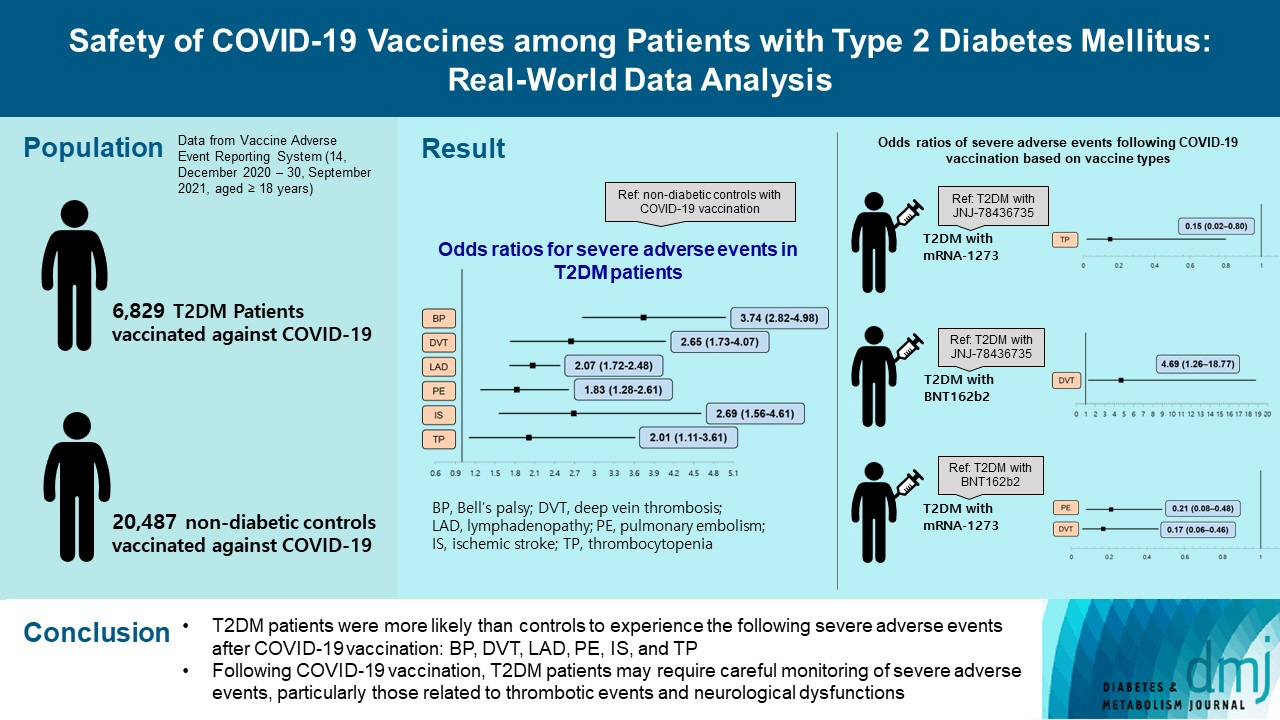
This study used vaccine AE reporting system data to investigate severe AEs among vaccinated patients with T2DM. A natural language processing algorithm was applied to identify people with and without diabetes. After 1:3 matching, we collected data for 6,829 patients with T2DM and 20,487 healthy controls. Multiple logistic regression analysis was used to calculate the odds ratio for severe AEs.
Results
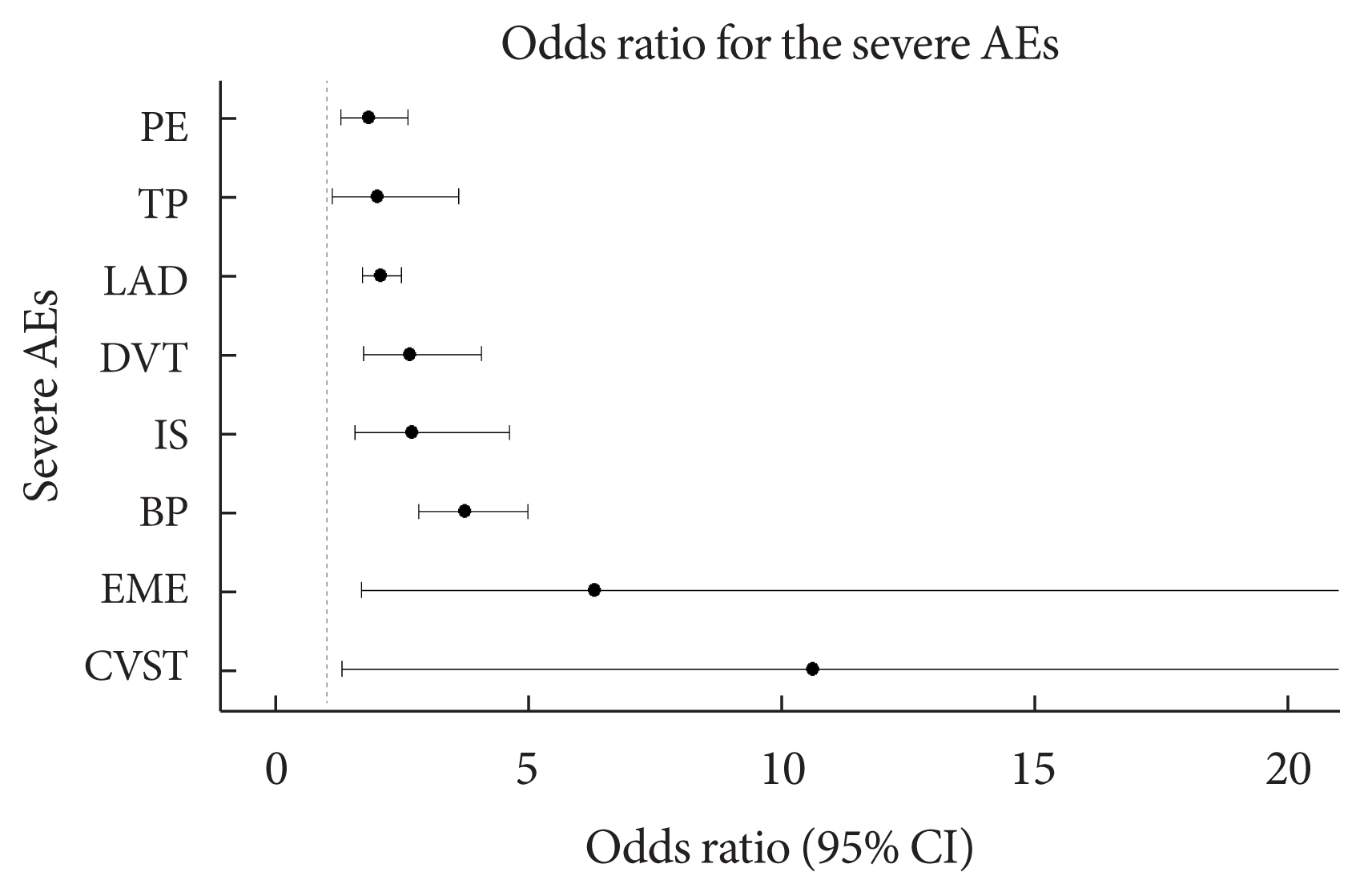
After COVID-19 vaccination, patients with T2DM were more likely to experience eight severe AEs than controls: cerebral venous sinus thrombosis, encephalitis myelitis encephalomyelitis, Bell’s palsy, lymphadenopathy, ischemic stroke, deep vein thrombosis (DVT), thrombocytopenia (TP), and pulmonary embolism (PE). Moreover, patients with T2DM vaccinated with BNT162b2 and mRNA-1273 were more vulnerable to DVT and TP than those vaccinated with JNJ-78436735. Among patients with T2DM administered mRNA vaccines, mRNA-1273 was safer than BNT162b2 in terms of the risk of DVT and PE.
Conclusion
Careful monitoring of severe AEs in patients with T2DM may be necessary, especially for those related to thrombotic events and neurological dysfunctions after COVID-19 vaccination.
Keyword
Figure
Reference
-
1. Cines DB, Bussel JB. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021; 384:2254–6.
Article2. Self WH, Tenforde MW, Rhoads JP, Gaglani M, Ginde AA, Douin DJ, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions: United States, March–August 2021. MMWR Morb Mortal Wkly Rep. 2021; 70:1337–43.3. Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021; 326:1390–9.4. Amanzio M, Mitsikostas DD, Giovannelli F, Bartoli M, Cipriani GE, Brown WA. Adverse events of active and placebo groups in SARS-CoV-2 vaccine randomized trials: a systematic review. Lancet Reg Health Eur. 2022; 12:100253.
Article5. Targher G, Mantovani A, Wang XB, Yan HD, Sun QF, Pan KH, et al. Patients with diabetes are at higher risk for severe illness from COVID-19. Diabetes Metab. 2020; 46:335–7.6. Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Obes Metab. 2021; 23:870–4.
Article7. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020; 14:395–403.
Article8. Al-Maqbali JS, Al Rasbi S, Kashoub MS, Al Hinaai AM, Farhan H, Al Rawahi B, et al. A 59-year-old woman with extensive deep vein thrombosis and pulmonary thromboembolism 7 days following a first dose of the Pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine. Am J Case Rep. 2021; 22:e932946.9. Ahmadi M, Rezaei Z, Shirazi FA, Shafiei M. Guillain–Barre syndrome: a prevalent autoimmune disease during the coronavirus disease-2019 pandemic. Rev Res Med Microbiol. 2022; 33:e198–211.
Article10. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015; 33:4398–405.
Article11. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019; 42(Suppl 1):S90–102.12. Python Software Foundation. re–Regular expression operations. Available from: https://docs.python.org/3/library/re.html (cited 2022 Aug 31).13. Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021; 385:1078–90.14. Merchant HA. COVID vaccines and thrombotic events: EMA issued warning to patients and healthcare professionals. J Pharm Policy Pract. 2021; 14:32.
Article15. Centers for Disease Control and Prevention. What is venous thromboembolism? Available from: https://www.cdc.gov/ncbddd/dvt/facts.html(cited 2022 Aug 31).16. Sookaromdee P, Wiwanitkit V. Change of safety interval from hyperviscosity problem in COVID-19 vaccine recipient with underlying diabetic problem. Indian J Endocrinol Metab. 2021; 25:257.
Article17. Joob B, Wiwanitkit V. Expected viscosity after COVID-19 vaccination, hyperviscosity and previous COVID-19. Clin Appl Thromb Hemost. 2021; 27:10760296211020833.
Article18. Mehta J, Singhal S. Hyperviscosity syndrome in plasma cell dyscrasias. Semin Thromb Hemost. 2003; 29:467–71.19. Cho YI, Mooney MP, Cho DJ. Hemorheological disorders in diabetes mellitus. J Diabetes Sci Technol. 2008; 2:1130–8.
Article20. Devi S, Mohakud S, Kar N, Muthuvel D. Deep vein thrombosis with pulmonary thromboembolism in a case of severe COVID-19 pneumonia. BMJ Case Rep. 2021; 14:e240932.
Article21. Khan IH, Savarimuthu S, Leung MS, Harky A. The need to manage the risk of thromboembolism in COVID-19 patients. J Vasc Surg. 2020; 72:799–804.22. Maddaloni E, Buzzetti R. COVID-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020; 36:e33213321.
Article23. Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020; 162:108132.
Article24. Klok FA, Kruip MJ, van der Meer NJ, Arbous MS, Gommers DA, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020; 191:145–7.
Article25. Kahn LS, McIntyre RS, Rafalson L, Berdine DE, Fox CH. Fasting blood glucose and depressive mood among patients with mental illness in a medicaid managed care program. Depress Res Treat. 2011; 2011:862708.
Article26. Atallah B, Mallah SI, AlMahmeed W. Anticoagulation in COVID-19. Eur Heart J Cardiovasc Pharmacother. 2020; 6:260–1.27. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2021; 51:1107–10.28. Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020; 99:1205–8.
Article29. Tu W, Gierada DS, Joe BN. COVID-19 vaccination-related lymphadenopathy: what to be aware of. Radiol Imaging Cancer. 2021; 3:e210038.30. Sampsonas F, Lagadinou M, Karampitsakos T, Solomou E, Doulberis M, Marangos M, et al. Prevalence and significance of mediastinal lymphadenopathy in patients with severe acute respiratory syndrome corona virus-2 infection. Eur Rev Med Pharmacol Sci. 2021; 25:3607–9.31. Wang Z, Wang Z. Identification of risk factors for in-hospital death of COVID-19 pneumonia: lessions from the early outbreak. BMC Infect Dis. 2021; 21:113.32. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77:683–90.
Article33. Karuppan MK, Devadoss D, Nair M, Chand HS, Lakshmana MK. SARS-CoV-2 infection in the central and peripheral nervous system-associated morbidities and their potential mechanism. Mol Neurobiol. 2021; 58:2465–80.
Article34. Afshar ZM, Babazadeh A, Afsharian M, Vaziri S, Ebrahimpour S. Bell’s palsy associated with COVID-19 infection: a case report. Oman Med J. 2021; 36:e313.
Article35. Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020; 7:001691.36. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020; 525:135–40.
Article37. Ghosh R, Roy D, Mandal A, Pal SK, Chandra Swaika B, Naga D, et al. Cerebral venous thrombosis in COVID-19. Diabetes Metab Syndr. 2021; 15:1039–45.
Article38. Barros A, Queiruga-Pineiro J, Lozano-Sanroma J, Alcalde I, Gallar J, Fernandez-Vega Cueto L, et al. Small fiber neuropathy in the cornea of COVID-19 patients associated with the generation of ocular surface disease. Ocul Surf. 2022; 23:40–8.
Article39. Cure E, Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes Metab Syndr. 2020; 14:349–50.
Article40. Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020; 127:104354.
Article41. Muir KL, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med. 2021; 384:1964–5.
Article42. Warkentin TE, Cuker A. COVID-19 Vaccine-induced immune thrombotic thrombocytopenia (VITT). Available from: https://www.uptodate.com/contents/covid-19-vaccine-induced-immune-thrombotic-thrombocytopenia-vitt(cited 2022 Aug 31).43. Takuva S, Takalani A, Garrett N, Goga A, Peter J, Louw V, et al. Thromboembolic events in the South African Ad26.COV2.S Vaccine Study. N Engl J Med. 2021; 385:570–1.
Article44. Kemper M, Berssenbrugge C, Lenz G, Mesters RM. Vaccine-induced pseudothrombocytopenia after Ad26.COV2.S vaccination. Ann Hematol. 2022; 101:927–8.45. Netherlands Pharmacovigilance Centre Lareb. Overview of thrombo-embolic events with COVID-19 vaccines. Available from: https://www.lareb.nl/media/yhbp4bxl/signal_oe_thromboembolic_events_j07bx_20210426_finalc.pdf(cited 2022 Aug 31).46. El-Sayed MS, Wechie GN, Low CS, Adesanya O, Rao N, Leung VJ. The incidence and duration of COVID-19 vaccine-related reactive lymphadenopathy on 18F-FDG PET-CT. Clin Med (Lond). 2021; 21:e633–8.47. Chen G, Li X, Sun M, Zhou Y, Yin M, Zhao B, et al. COVID-19 mRNA vaccines are generally safe in the short term: a vaccine vigilance real-world study says. Front Immunol. 2021; 12:669010.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety of COVID-19 Vaccines among Patients with Type 2 Diabetes Mellitus: Real-World Data Analysis (Diabetes Metab J 2023;47:356-65)
- Safety of COVID-19 Vaccines among Patients with Type 2 Diabetes Mellitus: Real-World Data Analysis (Diabetes Metab J 2023;47:356-65)
- Efficacy and Safety of COVID-19 Vaccines in Children Aged 5 to 11 Years: A Systematic Review
- Glucose-Lowering Agents and COVID-19
- Adult-Onset Type 1 Diabetes Development Following COVID-19 mRNA Vaccination