J Korean Med Sci.
2023 May;38(19):e143. 10.3346/jkms.2023.38.e143.
Effectiveness of Heterologous COVID-19 Vaccine Booster in Korean Elderly Population, 2022
- Affiliations
-
- 1Korea Disease Control and Prevention Agency, Cheongju, Korea
- 2Korea University Anam Hospital and Allergy and Immunology Center, Korea University, Seoul, Korea
- KMID: 2542394
- DOI: http://doi.org/10.3346/jkms.2023.38.e143
Abstract
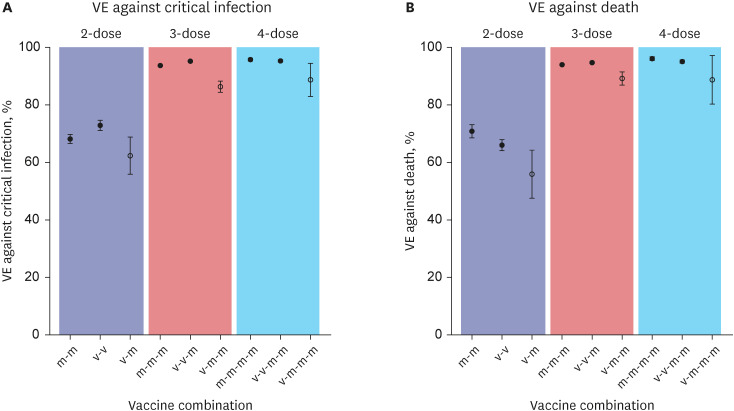
- We conducted a cohort study to assess vaccine effectiveness (VE) of coronavirus disease 2019 vaccine combinations on severe acute respirator y syndrome coronavirus 2 critical infection and death among elderly population in Korea. From Januar y to August 2022, VE against death for 4 doses mRNA recipients was 96.1%, whereas 1-dose viral vector + 3-dose mRNA recipients had VE of 90.8%.
Keyword
Figure
Reference
-
1. Shi HJ, Nham E, Kim B, Joo EJ, Cheong HS, Hong SH, et al. Clinical Characteristics and Risk Factors for Mortality in Critical Coronavirus Disease 2019 Patients 50 Years of Age or Younger During the Delta Wave: Comparison With Patients > 50 Years in Korea. J Korean Med Sci. 2022; 37(22):e175. PMID: 35668685.2. Kim YY, Choe YJ, Kim J, Kim RK, Jang EJ, Lee H, et al. Vaccine Effectiveness Against Severe Disease and Death for Patients With COVID-19 During the Delta-Dominant and Omicron-Emerging Periods: A K-COVE Study. J Korean Med Sci. 2023; 38(11):e87. PMID: 36942395.3. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022; 28(2):202–221. PMID: 34715347.4. Yi S, Choe YJ, Lim DS, Lee HR, Kim J, Kim YY, et al. Impact of national Covid-19 vaccination Campaign, South Korea. Vaccine. 2022; 40(26):3670–3675. PMID: 35570077.5. Diaz-Quijano FA. A simple method for estimating relative risk using logistic regression. BMC Med Res Methodol. 2012; 12(1):14. PMID: 22335836.6. Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021; 27(9):1530–1535. PMID: 34312554.7. Au WY, Cheung PP. Effectiveness of heterologous and homologous covid-19 vaccine regimens: living systematic review with network meta-analysis. BMJ. 2022; 377:e069989. PMID: 35640925.8. Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021; 398(10295):121–130. PMID: 34181880.9. Garg I, Sheikh AB, Pal S, Shekhar R. Mix-and-match COVID-19 vaccinations (heterologous boost): a review. Infect Dis Rep. 2022; 14(4):537–546. PMID: 35893476.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Herpes zoster ophthalmicus after COVID-19 vaccine booster in healthy younger adult: a case report
- A Case Report of MPO-ANCAAssociated Vasculitis Following Heterologous mRNA1273 COVID-19 Booster Vaccination
- Heterologous Vaccination for Preparing COVID-19 Pandemic
- Changes in SARS-CoV-2 antibody titers 6 months after the booster dose of BNT162b2 COVID-19 vaccine among health care workers
- Nasal vaccine as a booster shot: a viable solution to restrict pandemic?