Obstet Gynecol Sci.
2023 May;66(3):241-251. 10.5468/ogs.22280.
Use of contraceptive implants at 12 months in women who intended to undergo immediate versus delayed postpartum insertion following high-risk pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
- KMID: 2542230
- DOI: http://doi.org/10.5468/ogs.22280
Abstract
Objective
To assess the correlation between the intention to undergo immediate versus delayed postpartum contraceptive implant insertion following high-risk pregnancy, and the proportion of utilization and adverse effects.
Methods
We conducted a retrospective cohort study of women who gave birth after a high-risk pregnancy (according to the criteria defined by the Society for Maternal-Fetal Medicine) and intended to use contraceptive implants. The participants were classified into two groups based on whether they underwent immediate or delayed insertion. The primary outcome was the proportion of utilization of contraceptive implants at 12 months postpartum. We performed multivariate analyses to determine the relationships between the timing of insertion, characteristics, and methods used.
Results
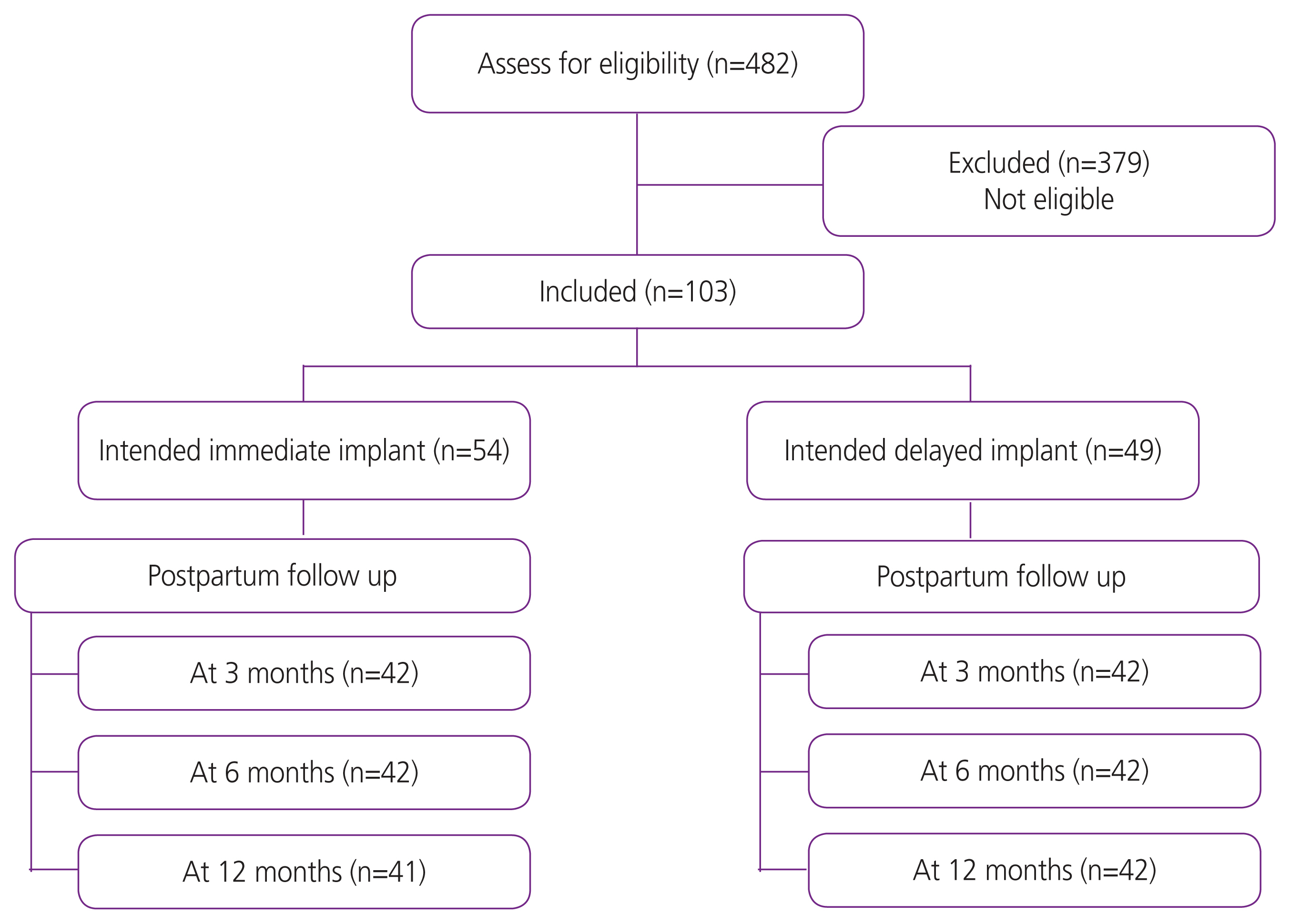
Of the 482 women classified as having high-risk pregnancies, 103 intended to use contraceptive implants (54 immediate and 49 delayed insertions). Women in the immediate group were more likely to use contraceptive implants than those in the delayed group at 6 (95.2% vs. 26.2%, P<0.01) and 12 months (92.7% vs. 26.2, P<0.01). A higher proportion of participants in the immediate group reported spotting and prolonged bleeding at 12 months (51.1% vs. 23.8%, P=0.01 and 26.8% vs. 7.1%, P=0.01; respectively). However, satisfaction at 12 months was higher in the immediate group than in the delayed group.
Conclusion
Intention to undergo implant insertion during the immediate postpartum period appears to improve the utilization of highly effective contraception. Patients who underwent immediate implantation experienced more spotting, prolonged bleeding, and dysmenorrhea. This study supports the recommendation to provide immediate postpartum contraceptive implants to women following high-risk pregnancies.
Keyword
Figure
Reference
-
References
1. Blackwell S, Louis JM, Norton ME, Lappen JR, Pettker CM, Kaimal A, et al. Reproductive services for women at high risk for maternal mortality: a report of the workshop of the society for maternal-fetal medicine, the American College of Obstetricians and Gynecologists, the Fellowship in Family Planning, and the Society of Family Planning. Am J Obstet Gynecol. 2020; 222:B2–18.
Article2. Chor J, Rankin K, Harwood B, Handler A. Unintended pregnancy and postpartum contraceptive use in women with and without chronic medical disease who experienced a live birth. Contraception. 2011; 84:57–63.
Article3. Vricella LK, Gawron LM, Louis JM. Society for maternal-fetal medicine (SMFM) consult series #48: immediate postpartum long-acting reversible contraception for women at high risk for medical complications. Am J Obstet Gynecol. 2019; 220:B2–12.
Article4. American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine. Obstetric care consensus No. 8: interpregnancy care. Obstet Gynecol. 2019; 133:e51–72.5. Committee on Practice Bulletins-Gynecology, Long-Acting Reversible Contraception Work Group. Practice bulletin No. 186: long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017; 130:e251–69.6. Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 2016; 65:1–103.
Article7. Jaruamornjit Y, Kaewrudee S, Sothornwit J. Differences in postpartum contraceptive choices and patterns following low- and high-risk pregnancy. Contraception. 2022; 107:52–7.
Article8. Sothornwit J, Kaewrudee S, Lumbiganon P, Pattanittum P, Averbach SH. Immediate versus delayed postpartum insertion of contraceptive implant and IUD for contraception. Cochrane Database Syst Rev. 2022; 10:CD01. 1913.
Article9. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007; 370:1453–7.
Article10. World Health Organization. Family planning: a global handbook for providers: evidence-based guidance developed through worldwide collaboration [Internet]. Geneva: WHO;c2018. [cited 2022 Sep 11]. Available from: https://apps.who.int/iris/bitstream/handle/10665/260156/9780999203705-eng.pdf?sequence=1 .11. Creinin MD, Vieira CS, Westhoff CL, Mansour DJA. Recommendations for standardization of bleeding data analyses in contraceptive studies. Contraception. 2022; 112:14–22.
Article12. Averbach S, Kakaire O, Kayiga H, Lester F, Sokoloff A, Byamugisha J, et al. Immediate versus delayed postpartum use of levonorgestrel contraceptive implants: a randomized controlled trial in Uganda. Am J Obstet Gynecol. 2017; 217:568e1–568.e7.
Article13. Kiykac Altinbas S, Bayoglu Tekin Y, Dilbaz B, Kilic S, Khalil SS, Kandemir O. Impact of having a high-risk pregnancy on future postpartum contraceptive method choice. Women Birth. 2014; 27:254–8.
Article14. Carmo LSMP, Braga GC, Ferriani RA, Quintana SM, Vieira CS. Timing of etonogestrel-releasing implants and growth of breastfed infants: a randomized controlled trial. Obstet Gynecol. 2017; 130:100–7.15. Bryant AG, Bauer AE, Stuart GS, Levi EE, Zerden ML, Danvers A, et al. Etonogestrel-releasing contraceptive implant for postpartum adolescents: a randomized controlled trial. J Pediatr Adolesc Gynecol. 2017; 30:389–94.
Article16. Lazenby G, Francis E, Brzozowski N, Rucker L, Dempsey A. Postpartum LARC discontinuation and short interval pregnancies among women with HIV: a retrospective 9-year cohort study in South Carolina. Contraception. 2019; 100:279–82.
Article17. Rodriguez MI, Skye M, Samandari G, Darney BG. Timing of postpartum long acting, reversible contraception was not associated with 12-month removal rates in a large Medicaid sample. Contraception. 2022; 113:49–56.
Article18. Hameed W, Azmat SK, Ali M, Ishaque M, Abbas G, Munroe E, et al. Comparing effectiveness of active and passive client follow-up approaches in sustaining the continued use of long acting reversible contraceptives (LARC) in rural punjab: a multicentre, non-inferiority trial. PLoS One. 2016; 11:e0160683.
Article19. Ireland LD, Goyal V, Raker CA, Murray A, Allen RH. The effect of immediate postpartum compared to delayed postpartum and interval etonogestrel contraceptive implant insertion on removal rates for bleeding. Contraception. 2014; 90:253–8.
Article20. Sappan R, Wattanakamolchai P, Werawatakul Y, Sothornwit J. Discontinuation of contraceptive implants within 12 months of use. Thai J Obstet Gynaecol. 2021; 29:198–207.21. Phemister DA, Laurent S, Harrison FN Jr. Use of Norplant contraceptive implants in the immediate postpartum period: safety and tolerance. Am J Obstet Gynecol. 1995; 172:175–9.
Article22. Kennedy KI, Short RV, Tully MR. Premature introduction of progestin-only contraceptive methods during lactation. Contraception. 1997; 55:347–50.
Article23. Averbach S, Kakaire O, McDiehl R, Dehlendorf C, Lester F, Steinauer J. The effect of immediate postpartum levonorgestrel contraceptive implant use on breastfeeding and infant growth: a randomized controlled trial. Contraception. 2019; 99:87–93.
Article24. Furman L, Shaker M, Arora KS. Do expectant mothers’ breastfeeding plans influence provider prenatal contraceptive counseling? J Hum Lact. 2020; 36:808–15.
Article25. Johnson NA, Fuell Wysong E, Tossone K, Furman L. Associations between prenatal intention and postpartum choice: infant feeding and contraception decisions among inner-city women. Breastfeed Med. 2019; 14:456–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recurrent Hyperthyroidism Following Postpartum Thyroiditis in a Woman with Hashimoto's Thyroiditis
- Postpartum modern contraceptive use in northern Ethiopia: prevalence and associated factors
- Removal of migrated subdermal contraceptive implant (Implanon) to axillar: A case report
- Prevalence of and Risk Factors for Depressive Symptoms in Korean Women throughout Pregnancy and in Postpartum Period
- Common Mental Disorders and Associated Factors During Pregnancy and the Postpartum Period in Indonesia: An Analysis of Data From the 2018 Basic Health Research