Obstet Gynecol Sci.
2023 May;66(3):190-197. 10.5468/ogs.23031.
Long term renal outcome after hypertensive disease during pregnancy: a nationwide population-based study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Obstetrics and Gynecology, Seoul National University Hospital, Seoul, Korea
- 3Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea
- KMID: 2542225
- DOI: http://doi.org/10.5468/ogs.23031
Abstract
Objective
Hypertensive disease during pregnancy increases the risk of maternal morbidity and mortality and leads to the development of multi-organ dysfunction, including kidney dysfunction. Complicated pregnancies require careful postpartum management to prevent sequelae. It is believed that kidney injury can consistently occur even after delivery; therefore, defining the chronicity and endpoint is essential for establishing diagnostic criteria. However, data on the prevalence of persistent renal complications following hypertensive disease during pregnancy are limited. In this study, we evaluated the risk of developing renal disorders in patients with a history of hypertensive disease during pregnancy.
Methods
Participants who gave birth between 2009 and 2010 were followed up for 8 years after delivery. The risk of renal disorder development after delivery was determined according to a history of hypertensive disease during pregnancy. Different factors that could affect the course of pregnancy, including age, primiparity, multiple pregnancy, preexisting hypertension, pregestational diabetes, hypertensive disease during pregnancy, gestational diabetes, postpartum hemorrhage, and cesarean section, were adjusted for using the Cox hazard model.
Results
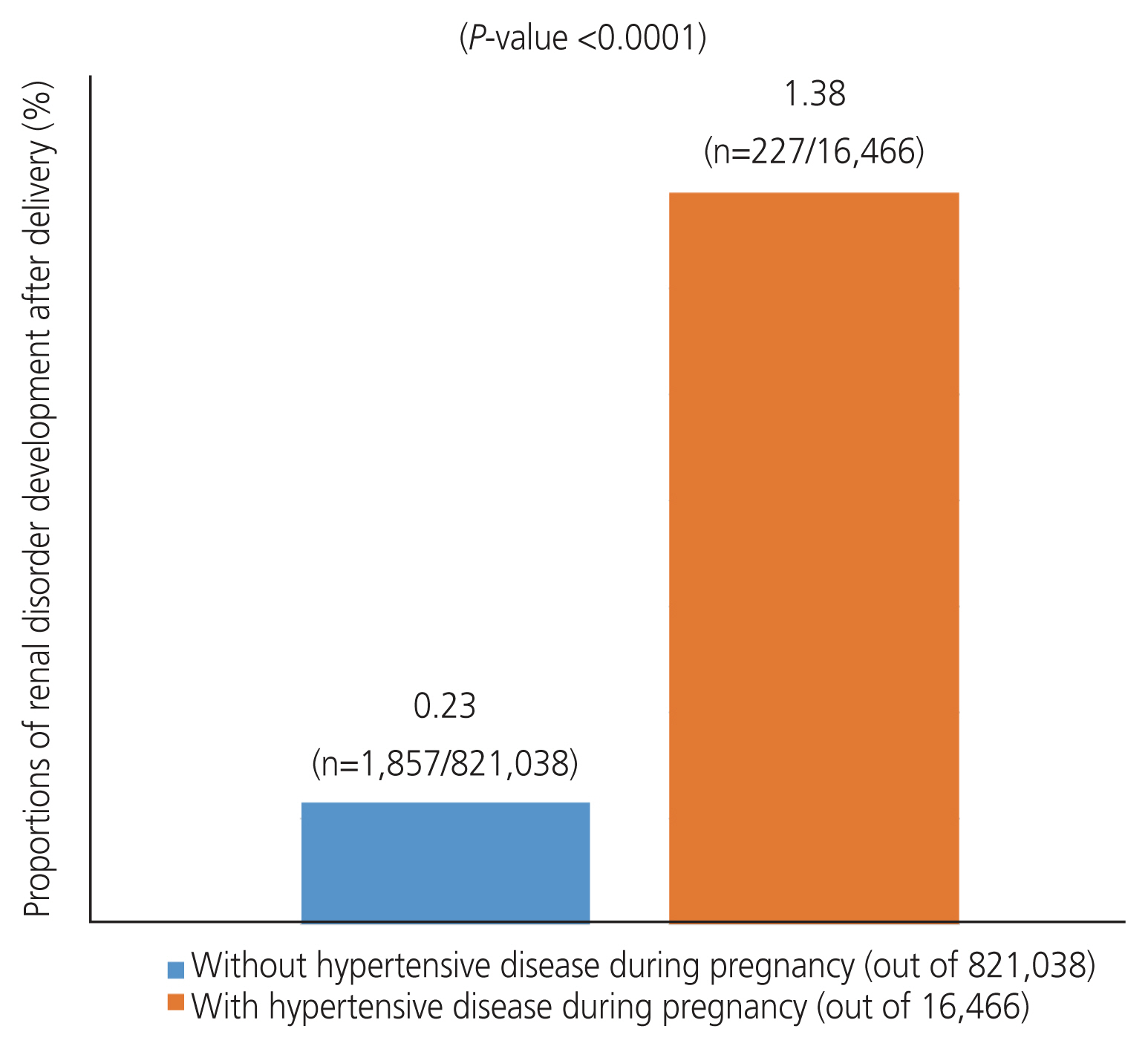
Women with hypertension during pregnancy had a higher risk of developing renal disorders after delivery (0.23% vs. 1.38%; P<0.0001). This increased risk remained significant even after adjusting for covariates (adjusted hazard ratio, 3.861; 95% confidence interval [CI], 3.400-4.385] and 4.209 [95% CI, 3.643-4.864]; respectively).
Conclusion
Hypertension during pregnancy can contribute to the development of renal disorders, even after delivery.
Keyword
Figure
Reference
-
References
1. Szczepanski J, Griffin A, Novotny S, Wallace K. Acute kidney injury in pregnancies complicated with preeclampsia or HELLP syndrome. Front Med (Lausanne). 2020; 7:22.
Article2. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013; 170:1–7.
Article3. Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008; 359:800–9.
Article4. Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019; 365:l1516.
Article5. Sandvik MK, Hallan S, Svarstad E, Vikse BE. Preeclampsia and prevalence of microalbuminuria 10 years later. Clin J Am Soc Nephrol. 2013; 8:1126–34.
Article6. Mangos GJ, Spaan JJ, Pirabhahar S, Brown MA. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens. 2012; 30:351–8.
Article7. Berks D, Steegers EAP, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol. 2009; 114:1307–14.
Article8. Dreyer G, Hull S, Aitken Z, Chesser A, Yaqoob MM. The effect of ethnicity on the prevalence of diabetes and associated chronic kidney disease. QJM. 2009; 102:261–9.
Article9. Peralta CA, Katz R, DeBoer I, Ix J, Sarnak M, Kramer H, et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol. 2011; 22:1327–34.
Article10. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014; 38:395–403.
Article11. Paauw ND, Luijken K, Franx A, Verhaar MC, Lely AT. Long-term renal and cardiovascular risk after preeclampsia: towards screening and prevention. Clin Sci (Lond). 2016; 130:239–46.
Article12. Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007; 2:543–9.
Article13. Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015; 309:R62–70.
Article14. Lopes van Balen VA, Spaan JJ, Cornelis T, Spaanderman MEA. Prevalence of chronic kidney disease after preeclampsia. J Nephrol. 2017; 30:403–9.
Article15. Barrett PM, McCarthy FP, Evans M, Kublickas M, Perry IJ, Stenvinkel P, et al. Does gestational diabetes increase the risk of maternal kidney disease? A Swedish national cohort study. PLoS One. 2022; 17:e0264992.
Article16. McDonald SD, Han Z, Walsh MW, Gerstein HC, Devereaux PJ. Kidney disease after preeclampsia: a systematic review and meta-analysis. Am J Kidney Dis. 2010; 55:1026–39.
Article17. Kovesdy CP, Furth S, Zoccali C; World Kidney Day Steering Committee. Obesity and kidney disease: hidden consequences of the epidemic. Physiol Int. 2017; 104:1–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fetal and Maternal Outcomes of Pregnancy in Women with IgA Nephropathy
- Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications
- Comparative study between pregnancies with and without hypertensive disorders in placental abruption
- Clinical Observation on the Effect of Parenteral Reserpine
- Does Pregnancy after Renal Transplantation Affect Their Allograft and Pregnancy Outcomes?