J Yeungnam Med Sci.
2023 Apr;40(2):164-171. 10.12701/jyms.2022.00276.
Effect of prehydration solution on hearing threshold after chemotherapy in patients with head and neck cancers: a retrospective study
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2541942
- DOI: http://doi.org/10.12701/jyms.2022.00276
Abstract
- Background
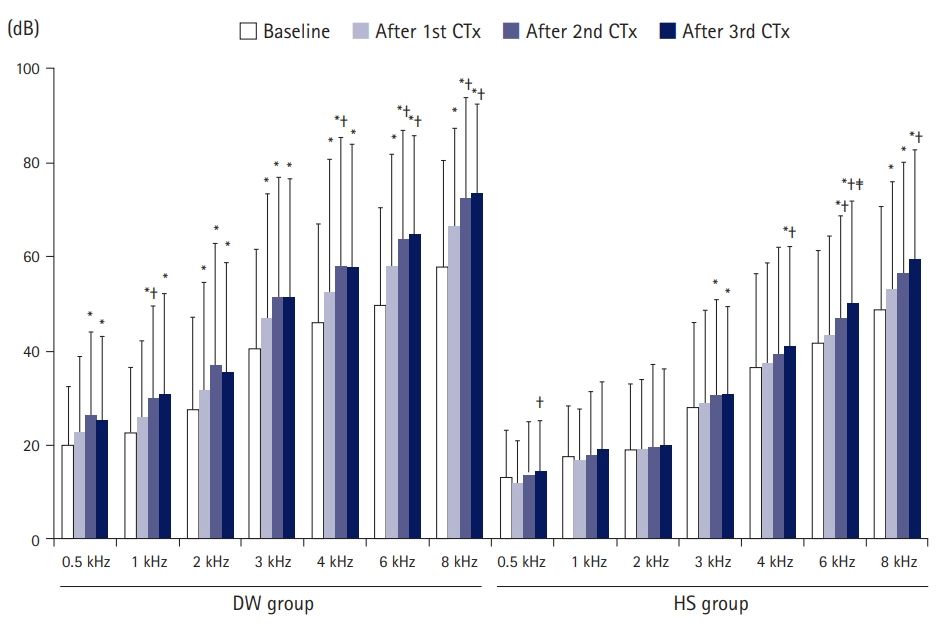
The study aimed to evaluate the effect of prehydration solution on hearing thresholds after cisplatin chemotherapy.
Methods
In this retrospective cohort study, we reviewed the data of patients who underwent ≥3 courses of cisplatin-based chemotherapy for locally advanced head and neck cancers at a tertiary referral center (n=64). The dextrose solution (DW) group (n=26) received 2 L of normal saline and 1 L of 5% dextrose. The Hartmann solution (HS) group (n=38) received 2 L of normal saline and 1 L of HS. Hearing data were measured 1 day before starting the first course of chemotherapy, and again 20 days after the first, second, and third courses of chemotherapy. The severity of hearing loss was evaluated using the Common Terminology Criteria for Adverse Events (CTCAE).
Results
Thresholds at all frequencies after chemotherapy were greater in the DW group than in the HS group. The increase in thresholds in 1 to 4 kHz after the third course of chemotherapy was greater in the DW group than in the HS group. CTCAE grades after the second and third courses of chemotherapy were greater in the DW group than in the HS group. Logistic regression showed that the odds ratio for CTCAE grade 3 or 4 after the third course of chemotherapy in the DW group was 4.84 on univariate analysis.
Conclusion
Prehydration using a solution with salt was associated with a decrease in change in hearing thresholds after cisplatin chemotherapy in patients with head and neck cancers.
Keyword
Figure
Reference
-
References
1. Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965; 205:698–9.2. Laurell G, Bagger-Sjöbäck D. Dose-dependent inner ear changes after i.v. administration of cisplatin. J Otolaryngol. 1991; 20:158–67.3. Wu X, Li X, Song Y, Li H, Bai X, Liu W, et al. Allicin protects auditory hair cells and spiral ganglion neurons from cisplatin - induced apoptosis. Neuropharmacology. 2017; 116:429–40.4. Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017; 8:1654.5. Santos NA, Ferreira RS, Santos AC. Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem Toxicol. 2020; 136:111079.6. Horie S, Oya M, Nangaku M, Yasuda Y, Komatsu Y, Yanagita M, et al. Guidelines for treatment of renal injury during cancer chemotherapy 2016. Clin Exp Nephrol. 2018; 22:210–44.7. Earhart RH, Martin PA, Tutsch KD, Ertürk E, Wheeler RH, Bull FE. Improvement in the therapeutic index of cisplatin (NSC 119875) by pharmacologically induced chloruresis in the rat. Cancer Res. 1983; 43:1187–94.8. Daley-Yates PT, McBrien DC. A study of the protective effect of chloride salts on cisplatin nephrotoxicity. Biochem Pharmacol. 1985; 34:2363–9.9. Ahn D, Lee GJ, Sohn JH, Lee JE. Phase II trial of individualized/dynamic cisplatin regimens for definitive concurrent chemoradiation therapy in patients with head and neck squamous cell carcinoma. Cancer Med. 2020; 9:9256–65.10. Common Terminology Criteria for Adverse Events (CTCAE v5.0). U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; 2017 [cited 2022 Jun 5]. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50.11. Trendowski MR, El Charif O, Dinh PC Jr, Travis LB, Dolan ME. Genetic and modifiable risk factors contributing to cisplatin-induced toxicities. Clin Cancer Res. 2019; 25:1147–55.12. More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J Neurosci. 2010; 30:9500–9.13. Ciarimboli G. Membrane transporters as mediators of cisplatin side-effects. Anticancer Res. 2014; 34:547–50.14. Karasawa T, Steyger PS. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett. 2015; 237:219–27.15. Duval M, Daniel SJ. Meta-analysis of the efficacy of amifostine in the prevention of cisplatin ototoxicity. J Otolaryngol Head Neck Surg. 2012; 41:309–15.16. Freyer DR, Brock PR, Chang KW, Dupuis LL, Epelman S, Knight K, et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: a clinical practice guideline. Lancet Child Adolesc Health. 2020; 4:141–50.17. Paken J, Govender CD, Pillay M, Sewram V. Cisplatin-associated ototoxicity: a review for the health professional. J Toxicol. 2016; 2016:1809394.18. Paken J, Govender CD, Pillay M, Sewram V. A review of cisplatin-associated ototoxicity. Semin Hear. 2019; 40:108–21.19. Santoso JT, Lucci JA 3rd, Coleman RL, Schafer I, Hannigan EV. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: a randomized trial. Cancer Chemother Pharmacol. 2003; 52:13–8.20. Tang Q, Wang X, Jin H, Mi Y, Liu L, Dong M, et al. Cisplatin-induced ototoxicity: updates on molecular mechanisms and otoprotective strategies. Eur J Pharm Biopharm. 2021; 163:60–71.21. Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007; 33:9–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pure tone hearing threshold and maximum speech discrimination scorein sensori-neural hearing loss
- Noise-Induced and Age-Related Hearing Loss in Korea
- The Change of Hearing Threshold by the Lactate Delivered to Round Window in Guinea Pigs
- Analysis of the Latency of Auditory Brainstem Response Wave V in Infants According to Age, Hearing Threshold and Stimulus Intensity
- The Effect of Gingko Biloba on Hearing in Mice with Noise-Induced Temporary Threshold Shift