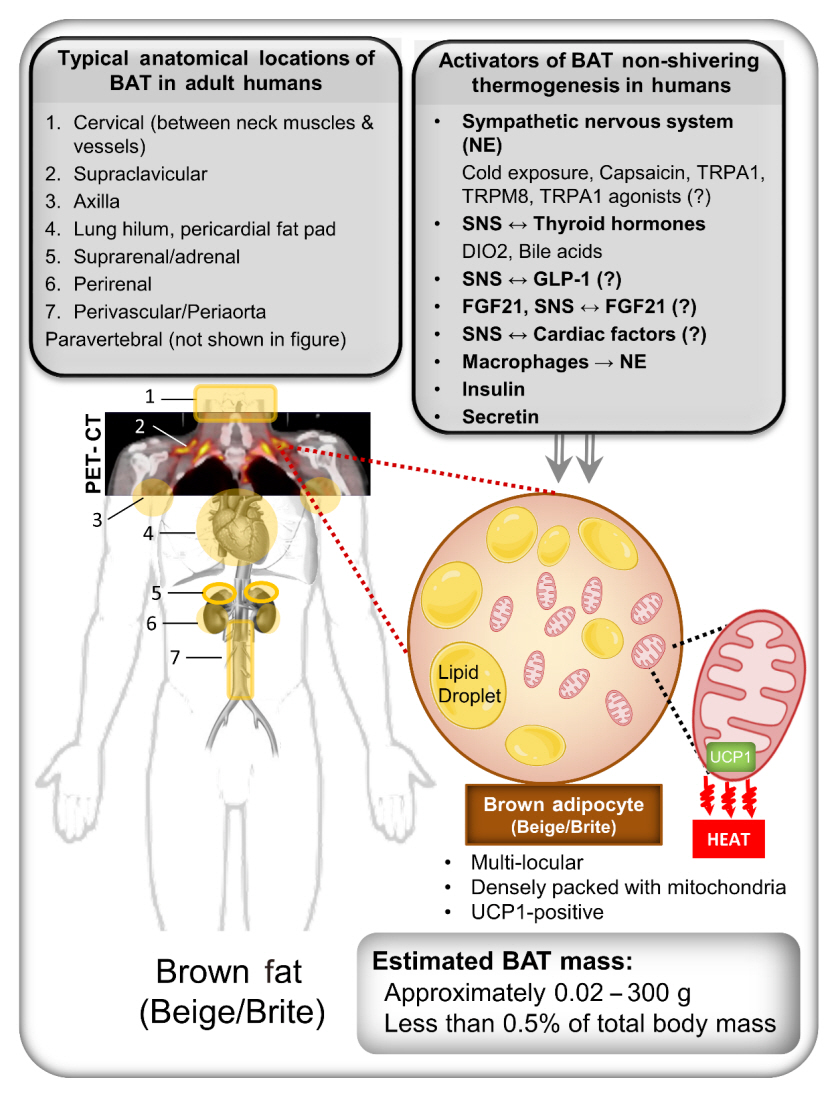
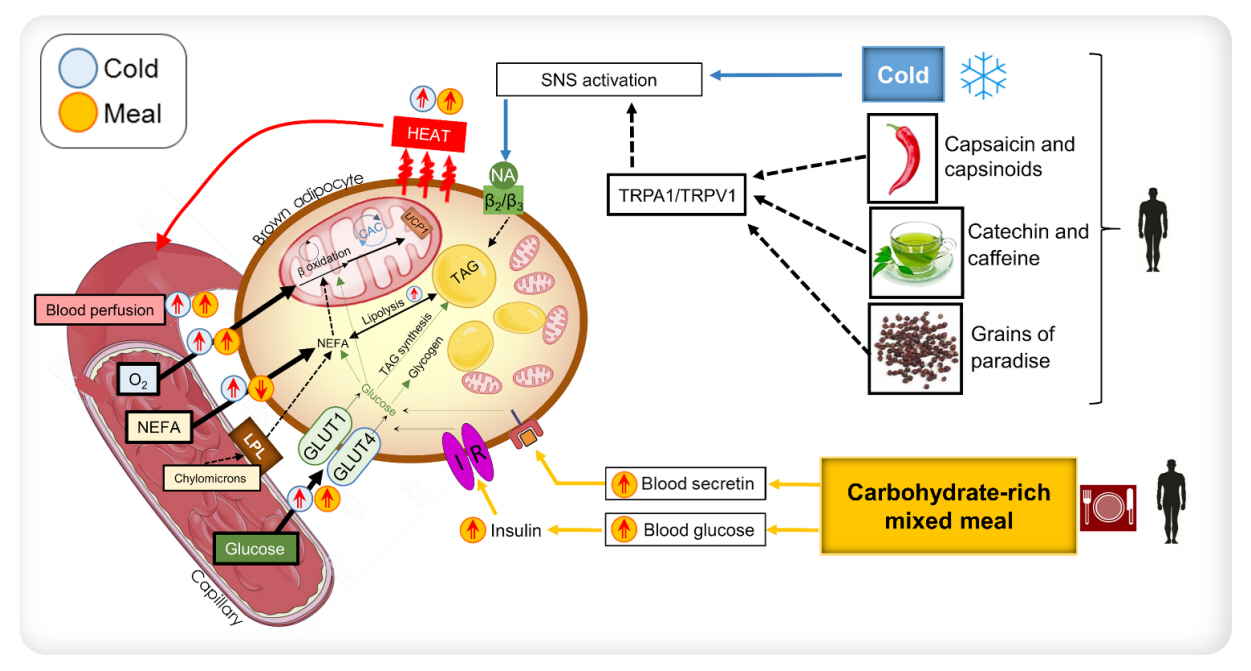
Endocrinol Metab.
2023 Apr;38(2):214-222. 10.3803/EnM.2023.1659.
Brown Adipose Tissue: Activation and Metabolism in Humans
- Affiliations
-
- 1Turku PET Center, Turku University Hospital, Turku, Finland
- 2Turku PET Centre, University of Turku, Turku, Finland
- KMID: 2541877
- DOI: http://doi.org/10.3803/EnM.2023.1659
Abstract
- Brown adipose tissue (BAT) is a thermogenic organ contributing to non-shivering thermogenesis. BAT becomes active under cold stress via sympathetic nervous system activation. However, recent evidence has suggested that BAT may also be active at thermoneutrality and in a postprandial state. BAT has superior energy dissipation capacity compared to white adipose tissue (WAT) and muscles. Thus, it has been proposed that the recruitment and activation of additional BAT may increase the overall energy-expending capacity in humans, potentially improving current whole-body weight management strategies. Nutrition plays a central role in obesity and weight management. Thus, this review discusses human studies describing BAT hyper-metabolism after dietary interventions. Nutritional agents that can potentially recruit brown adipocytes via the process of BAT-WAT transdifferentiation are also discussed.
Figure
Reference
-
1. Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005; 73:9–15.
Article2. Cinti S. The role of brown adipose tissue in human obesity. Nutr Metab Cardiovasc Dis. 2006; 16:569–74.
Article3. Lindberg O, de Pierre J, Rylander E, Afzelius BA. Studies of the mitochondrial energy-transfer system of brown adipose tissue. J Cell Biol. 1967; 34:293–310.
Article4. Hausberger FX, Widelitz MM. Distribution of labeled erythrocytes in adipose tissue and muscle in the rat. Am J Physiol. 1963; 204:649–52.
Article5. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004; 84:277–359.
Article6. Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond). 2010; 34 Suppl 1:S36–42.
Article7. Cinti S. The adipose organ at a glance. Dis Model Mech. 2012; 5:588–94.
Article8. Lean ME, James WP. Uncoupling protein in human brown adipose tissue mitochondria: isolation and detection by specific antiserum. FEBS Lett. 1983; 163:235–40.9. Kramarova TV, Shabalina IG, Andersson U, Westerberg R, Carlberg I, Houstek J, et al. Mitochondrial ATP synthase levels in brown adipose tissue are governed by the c-Fo subunit P1 isoform. FASEB J. 2008; 22:55–63.
Article10. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012; 150:366–76.
Article11. Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006; 296:164–76.12. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008; 454:961–7.
Article13. Ishibashi J, Seale P. Medicine: beige can be slimming. Science. 2010; 328:1113–4.14. Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007; 104:4401–6.
Article15. Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab. 2009; 10:324–35.
Article16. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010; 285:7153–64.17. Enerback S. The origins of brown adipose tissue. N Engl J Med. 2009; 360:2021–3.
Article18. Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes. 2010; 17:143–9.
Article19. Cinti S. Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab. 2009; 297:E977–86.
Article20. Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, “brite,” and white adipose tissues. Am J Physiol Endocrinol Metab. 2012; 302:E19–31.21. Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012; 7:e49452.
Article22. Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, et al. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013; 19:631–4.
Article23. Nedergaard J, Cannon B. UCP1 mRNA does not produce heat. Biochim Biophys Acta. 2013; 1831:943–9.
Article24. Aherne W, Hull D. Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol. 1966; 91:223–34.
Article25. Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972; 112(Pt 1):35–9.26. Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013; 62:1783–90.27. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009; 360:1509–17.
Article28. Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011; 14:272–9.29. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Coldactivated brown adipose tissue in healthy men. N Engl J Med. 2009; 360:1500–8.
Article30. Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med. 2013; 54:523–31.
Article31. U Din M, Raiko J, Saari T, Kudomi N, Tolvanen T, Oikonen V, et al. Human brown adipose tissue [15O]O2 PET imaging in the presence and absence of cold stimulus. Eur J Nucl Med Mol Imaging. 2016; 43:1878–86.
Article32. GerngroB C, Schretter J, Klingenspor M, Schwaiger M, Fromme T. Active brown fat during 18F-FDG PET/CT imaging defines a patient group with characteristic traits and an increased probability of brown fat redetection. J Nucl Med. 2017; 58:1104–10.
Article33. Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012; 122:545–52.
Article34. Smith RE. Thermoregulatory and adaptive behavior of brown adipose tissue. Science. 1964; 146:1686–9.
Article35. Mattu A, Brady WJ, Perron AD. Electrocardiographic manifestations of hypothermia. Am J Emerg Med. 2002; 20:314–26.
Article36. Glick Z, Teague RJ, Bray GA. Brown adipose tissue: thermic response increased by a single low protein, high carbohydrate meal. Science. 1981; 213:1125–7.
Article37. Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979; 281:31–5.
Article38. Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010; 11:263–7.
Article39. U Din M, Saari T, Raiko J, Kudomi N, Maurer SF, Lahesmaa M, et al. Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell Metab. 2018; 28:207–16.
Article40. Young JB, Saville E, Rothwell NJ, Stock MJ, Landsberg L. Effect of diet and cold exposure on norepinephrine turnover in brown adipose tissue of the rat. J Clin Invest. 1982; 69:1061–71.
Article41. Fares A. Winter cardiovascular diseases phenomenon. N Am J Med Sci. 2013; 5:266–79.
Article42. Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014; 114:1815–26.
Article43. Moreira FA, Crippa JA. The psychiatric side-effects of rimonabant. Braz J Psychiatry. 2009; 31:145–53.
Article44. Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 2013; 17:638–43.
Article45. Muzik O, Mangner TJ, Granneman JG. Assessment of oxidative metabolism in brown fat using PET imaging. Front Endocrinol (Lausanne). 2012; 3:15.
Article46. Hibi M, Oishi S, Matsushita M, Yoneshiro T, Yamaguchi T, Usui C, et al. Brown adipose tissue is involved in diet-induced thermogenesis and whole-body fat utilization in healthy humans. Int J Obes (Lond). 2016; 40:1655–61.
Article47. Sugita J, Yoneshiro T, Hatano T, Aita S, Ikemoto T, Uchiwa H, et al. Grains of paradise (Aframomum melegueta) extract activates brown adipose tissue and increases whole-body energy expenditure in men. Br J Nutr. 2013; 110:733–8.
Article48. Yoneshiro T, Matsushita M, Hibi M, Tone H, Takeshita M, Yasunaga K, et al. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am J Clin Nutr. 2017; 105:873–81.49. Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte EE. Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne). 2018; 9:447.
Article50. Blondin DP, Labbe SM, Phoenix S, Guerin B, Turcotte EE, Richard D, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015; 593:701–14.
Article51. Ma SW, Foster DO. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can J Physiol Pharmacol. 1986; 64:609–14.52. Weir G, Ramage LE, Akyol M, Rhodes JK, Kyle CJ, Fletcher AM, et al. Substantial metabolic activity of human brown adipose tissue during warm conditions and cold-induced lipolysis of local triglycerides. Cell Metab. 2018; 27:1348–55.
Article53. Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015; 21:33–8.
Article54. Townsend KL, Tseng YH. Brown fat fuel utilization and thermogenesis. Trends Endocrinol Metab. 2014; 25:168–77.
Article55. Li Y, Schnabl K, Gabler SM, Willershauser M, Reber J, Karlas A, et al. Secretin-activated brown fat mediates prandial thermogenesis to induce satiation. Cell. 2018; 175:1561–74.
Article56. Laurila S, Sun L, Lahesmaa M, Schnabl K, Laitinen K, Klen R, et al. Secretin activates brown fat and induces satiation. Nat Metab. 2021; 3:798–809.
Article57. Lahesmaa M, Orava J, Schalin-Jantti C, Soinio M, Hannukainen JC, Noponen T, et al. Hyperthyroidism increases brown fat metabolism in humans. J Clin Endocrinol Metab. 2014; 99:E28–35.
Article58. Ebner S, Burnol AF, Ferre P, de Saintaurin MA, Girard J. Effects of insulin and norepinephrine on glucose transport and metabolism in rat brown adipocytes: potentiation by insulin of norepinephrine-induced glucose oxidation. Eur J Biochem. 1987; 170:469–74.
Article59. Inokuma K, Ogura-Okamatsu Y, Toda C, Kimura K, Yamashita H, Saito M. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes. 2005; 54:1385–91.
Article60. Vosselman MJ, Brans B, van der Lans AA, Wierts R, van Baak MA, Mottaghy FM, et al. Brown adipose tissue activity after a high-calorie meal in humans. Am J Clin Nutr. 2013; 98:57–64.
Article61. Teruel T, Valverde AM, Benito M, Lorenzo M. Insulin-like growth factor I and insulin induce adipogenic-related gene expression in fetal brown adipocyte primary cultures. Biochem J. 1996; 319(Pt 2):627–32.
Article62. Klein J, Fasshauer M, Klein HH, Benito M, Kahn CR. Novel adipocyte lines from brown fat: a model system for the study of differentiation, energy metabolism, and insulin action. Bioessays. 2002; 24:382–8.
Article63. Omatsu-Kanbe M, Zarnowski MJ, Cushman SW. Hormonal regulation of glucose transport in a brown adipose cell preparation isolated from rats that shows a large response to insulin. Biochem J. 1996; 315(Pt 1):25–31.
Article64. Labbe SM, Caron A, Lanfray D, Monge-Rofarello B, Bartness TJ, Richard D. Hypothalamic control of brown adipose tissue thermogenesis. Front Syst Neurosci. 2015; 9:150.65. Sanchez-Alavez M, Tabarean IV, Osborn O, Mitsukawa K, Schaefer J, Dubins J, et al. Insulin causes hyperthermia by direct inhibition of warm-sensitive neurons. Diabetes. 2010; 59:43–50.
Article66. Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring). 2011; 19:13–6.
Article67. Sun L, Camps SG, Goh HJ, Govindharajulu P, Schaefferkoetter JD, Townsend DW, et al. Capsinoids activate brown adipose tissue (BAT) with increased energy expenditure associated with subthreshold 18-fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan. Am J Clin Nutr. 2018; 107:62–70.
Article68. Blondin DP, Labbe SM, Tingelstad HC, Noll C, Kunach M, Phoenix S, et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab. 2014; 99:E438–46.
Article69. Hanssen MJ, Hoeks J, Brans B, van der Lans AA, Schaart G, van den Driessche JJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015; 21:863–5.
Article70. Hanssen MJ, van der Lans AA, Brans B, Hoeks J, Jardon KM, Schaart G, et al. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes. 2016; 65:1179–89.
Article71. van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013; 123:3395–403.
Article72. Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013; 123:3404–8.
Article73. So B, Kim HJ, Kim J, Song W. Exercise-induced myokines in health and metabolic diseases. Integr Med Res. 2014; 3:172–9.
Article74. Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003; 43:89–143.
Article75. Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr. 1999; 70:1040–5.
Article76. Gosselin C, Haman F. Effects of green tea extracts on nonshivering thermogenesis during mild cold exposure in young men. Br J Nutr. 2013; 110:282–8.
Article77. Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, et al. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr. 2005; 81:122–9.
Article78. Nirengi S, Amagasa S, Homma T, Yoneshiro T, Matsumiya S, Kurosawa Y, et al. Daily ingestion of catechin-rich beverage increases brown adipose tissue density and decreases extramyocellular lipids in healthy young women. Springerplus. 2016; 5:1363.
Article79. Akendengue B, Louis AM. Medicinal plants used by the Masango people in Gabon. J Ethnopharmacol. 1994; 41:193–200.
Article80. Riera CE, Menozzi-Smarrito C, Affolter M, Michlig S, Munari C, Robert F, et al. Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br J Pharmacol. 2009; 157:1398–409.
Article81. Iwami M, Mahmoud FA, Shiina T, Hirayama H, Shima T, Sugita J, et al. Extract of grains of paradise and its active principle 6-paradol trigger thermogenesis of brown adipose tissue in rats. Auton Neurosci. 2011; 161:63–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Brown Fat and Browning for the Treatment of Obesity and Related Metabolic Disorders
- The Mechanism of White and Brown Adipocyte Differentiation
- Immune cells in brown adipose tissue are involved in allogeneic immune responses
- Recent advance in brown adipose physiology and its therapeutic potential
- Brown Fat as a Regulator of Systemic Metabolism beyond Thermogenesis