Subclassification of advanced-stage hepatocellular carcinoma with macrovascular invasion: combined transarterial chemoembolization and radiotherapy as an alternative first-line treatment
- Affiliations
-
- 1Department of Gastroenterology, Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Radiation Oncology, Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2540830
- DOI: http://doi.org/10.17998/jlc.2023.03.04
Abstract
- Background
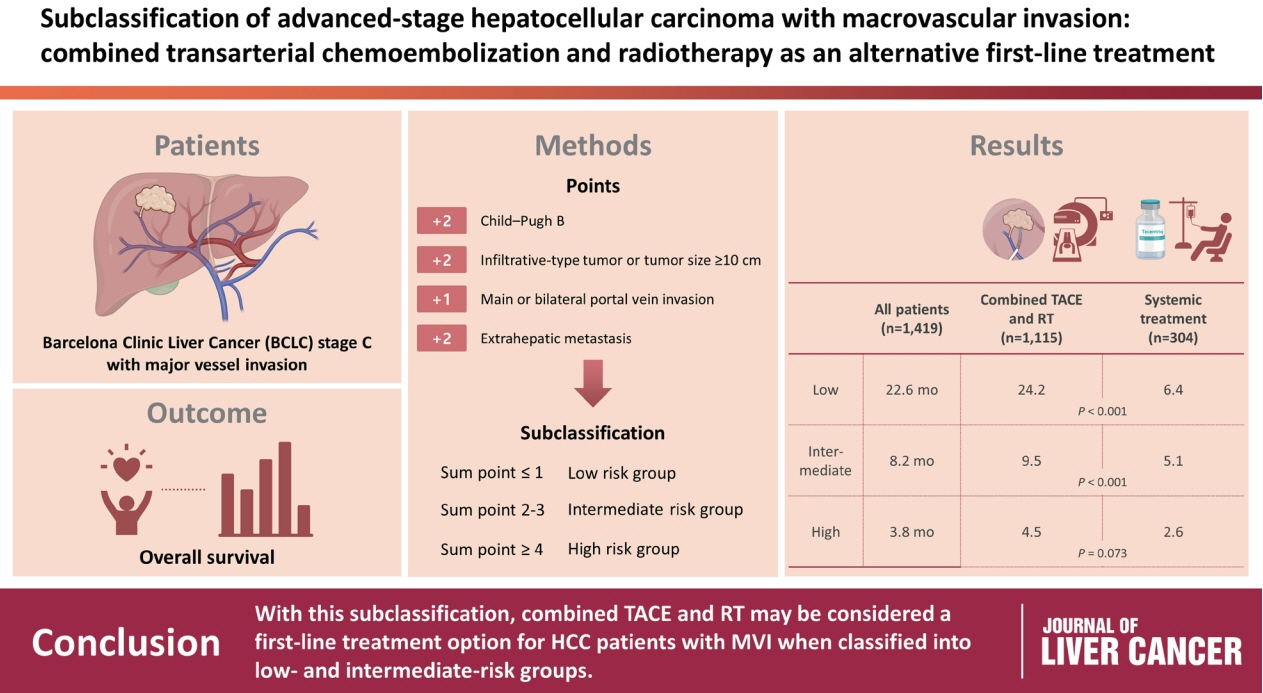
/Aim: The Barcelona Clinic Liver Cancer (BCLC) guidelines recommend systemic therapy as the only first-line treatment for patients with BCLC stage C hepatocellular carcinoma (HCC) despite its heterogeneity of disease extent. We aimed to identify patients who might benefit from combined transarterial chemoembolization (TACE) and radiation therapy (RT) by subclassifying BCLC stage C.
Methods
A total of 1,419 treatment-naïve BCLC stage C patients with macrovascular invasion (MVI) who were treated with combined TACE and RT (n=1,115) or systemic treatment (n=304) were analyzed. The primary outcome was overall survival (OS). Factors associated with OS were identified and assigned points by the Cox model. The patients were subclassified into three groups based on these points.
Results
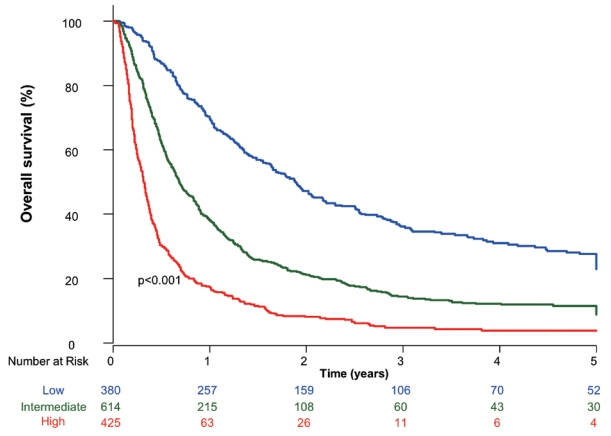
The mean age was 55.4 years, and 87.8% were male. The median OS was 8.3 months. Multivariate analysis revealed a significant association of Child-Pugh B, infiltrative-type tumor or tumor size ≥10 cm, main or bilateral portal vein invasion, and extrahepatic metastasis with poor OS. The sub-classification was categorized into low (point ≤1), intermediate (point=2), and high (point ≥3) risks based on the sum of points (range, 0–4). The OS in the low, intermediate, and high-risk groups was 22.6, 8.2, and 3.8 months, respectively. In the low and intermediate-risk groups, patients treated with combined TACE and RT exhibited significantly longer OS (24.2 and 9.5 months, respectively) than those who received systemic treatment (6.4 and 5.1 months, respectively; P<0.0001).
Conclusions
Combined TACE and RT may be considered as a first-line treatment option for HCC patients with MVI when classified into low- and intermediate-risk groups.
Figure
Cited by 4 articles
-
How to optimize the treatment strategy for advanced-stage hepatocellular carcinoma with macrovascular invasion
Beom Kyung Kim
J Liver Cancer. 2023;23(1):121-123. doi: 10.17998/jlc.2023.3.10.Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
J Liver Cancer. 2024;24(1):81-91. doi: 10.17998/jlc.2023.12.25.Liver resection in selective hepatocellular carcinoma with Vp3 or Vp4 portal vein tumor thrombosis improves prognosis
Manuel Lim, Jongman Kim, Jinsoo Rhu, Gyu-Seong Choi, Jae-Won Joh
J Liver Cancer. 2024;24(1):102-112. doi: 10.17998/jlc.2024.01.31.Management strategies for advanced hepatocellular carcinoma with portal vein tumor thrombosis
Jeayeon Park, Su Jong Yu
Ewha Med J. 2025;48(1):e4. doi: 10.12771/emj.2025.e4.
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999; 29:62–67.
Article3. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022; 76:681–693.
Article4. Tazawa J, Maeda M, Sakai Y, Yamane M, Ohbayashi H, Kakinuma S, et al. Radiation therapy in combination with transcatheter arterial chemoembolization for hepatocellular carcinoma with extensive portal vein involvement. J Gastroenterol Hepatol. 2001; 16:660–665.
Article5. Liu PH, Lee YH, Hsia CY, Hsu CY, Huang YH, Chiou YY, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol. 2014; 21:1825–1833.
Article6. Kim GA, Shim JH, Yoon SM, Jung J, Kim JH, Ryu MH, et al. Comparison of chemoembolization with and without radiation therapy and sorafenib for advanced hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. J Vasc Interv Radiol. 2015; 26:320–329.e6.
Article7. Kokudo T, Hasegawa K, Matsuyama Y, Takayama T, Izumi N, Kadoya M, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol. 2016; 65:938–943.
Article8. Yu JI, Park JW, Park HC, Yoon SM, Lim DH, Lee JH, et al. Clinical impact of combined transarterial chemoembolization and radiotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: an external validation study. Radiother Oncol. 2016; 118:408–415.
Article9. Lee D, Lee HC, An J, Shim JH, Kim KM, Lim YS, et al. Comparison of surgical resection versus transarterial chemoembolization with additional radiation therapy in patients with hepatocellular carcinoma with portal vein invasion. Clin Mol Hepatol. 2018; 24:144–150.
Article10. Kim YJ, Jung J, Joo JH, Kim SY, Kim JH, Lim YS, et al. Combined transarterial chemoembolization and radiotherapy as a first-line treatment for hepatocellular carcinoma with macroscopic vascular invasion: necessity to subclassify Barcelona Clinic Liver Cancer stage C. Radiother Oncol. 2019; 141:95–100.
Article11. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018; 4:661–669.
Article12. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.
Article13. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022; 1:1–12.
Article14. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.15. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.
Article16. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019; 13:227–299.17. Choi J, Lee D, Shim JH, Kim KM, Lim YS, Lee YS, et al. Evaluation of transarterial chemoembolization refractoriness in patients with hepatocellular carcinoma. PLoS One. 2020; 15:e0229696.
Article18. Lee S, Kim BK, Song K, Park JY, Ahn SH, Kim SU, et al. Subclassification of Barcelona Clinic Liver Cancer B and C hepatocellular carcinoma: a cohort study of the multicenter registry database. J Gastroenterol Hepatol. 2016; 31:842–847.
Article19. Yoo JJ, Chung GE, Lee JH, Nam JY, Chang Y, Lee JM, et al. Sub-classification of advanced-stage hepatocellular carcinoma: a cohort study including 612 patients treated with sorafenib. Cancer Res Treat. 2018; 50:366–373.
Article20. Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012; 57:821–829.
Article21. Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022; 76:862–873.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Transarterial Chemoembolization and External Beam Radiotherapy in a Patient with Recurrent Huge Hepatocellular Carcinoma after Hepatic Resection
- Evaluation of the Outcome after Transarterial Chemoembolization; Refinement of Barcelona Clinic Liver Cancer Stage-B from Eastern Point of View
- A Case of Small Hepatocellular Carcinoma Who Showed Complete Response by Combined Therapy of Transarterial Chemoembolization and Stereotactic Body Radiotherapy
- Comparison of surgical resection versus transarterial chemoembolization with additional radiation therapy in patients with hepatocellular carcinoma with portal vein invasion
- Complications Related to Transarterial Treatment of Hepatocellular Carcinoma: A Comprehensive Review