J Cerebrovasc Endovasc Neurosurg.
2023 Mar;25(1):62-68. 10.7461/jcen.2022.E2022.01.003.
Trapping and A4-A4 end-to-side anastomosis for the treatment of a ruptured A3 fusiform aneurysm: Potential risk of in-situ bypass
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2540808
- DOI: http://doi.org/10.7461/jcen.2022.E2022.01.003
Abstract
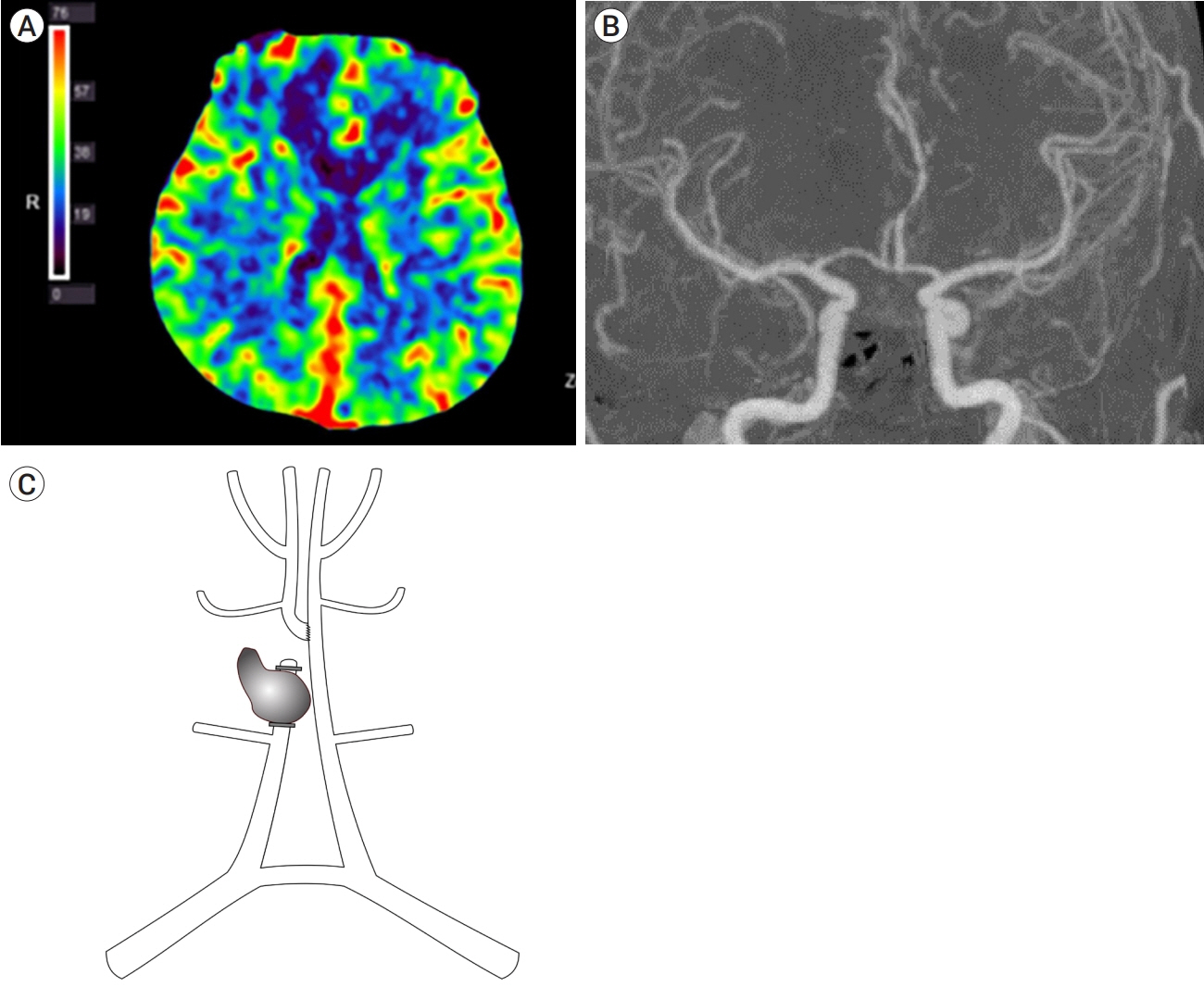
- The treatment of complicated anterior cerebral artery aneurysms remains challenging. Here, the authors describe a case of ruptured complicated A3 aneurysm, which was treated with trapping and in-situ bypass. A 47-year-old man presented to the emergency department with severe headache and vomiting. Computed tomography illustrated acute intracerebral hemorrhage in the right frontal lobe. Digital subtraction angiography (DSA) confirmed a ruptured fusiform A3 aneurysm with lobulation and a daughter sac. Trapping of the ruptured fusiform A3 aneurysm and distal end-toside A4 anastomosis was performed. DSA on postoperative day 7 showed mild vasospasm to the afferent artery. However, 2 months later, DSA demonstrated that the antegrade flow through the anastomosis site had recovered. Thus, surgeons should be aware of the possibility of postsurgical vasospasm of anastomosed arteries, especially in cases of ruptured aneurysms.
Figure
Reference
-
1. Abla AA, Lawton MT. Anterior cerebral artery bypass for complex aneurysms: an experience with intracranial-intracranial reconstruction and review of bypass options. J Neurosurg. 2014; Jun. 120(6):1364–77.2. Horiuchi T, Ichinose S, Agata M, Ito K, Hongo K. STA-ACA bypass using the ipsilateral free STA graft as an interposition graft and A3-A3 anastomosis for treatment of bilateral ACA steno-occlusive ischemia. Acta Neurochir (Wien). 2018; Apr. 160(4):779–82.3. Inagawa T, Yahara K, Ohbayashi N. Risk factors associated with cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo). 2014; Jun. 54(6):465–73.4. Lee SH, Ahn JS, Kwun BD, Park W, Park JC, Roh SW. Surgical flow alteration for the treatment of intracranial aneurysms that are unclippable, untrappable, and uncoilable. J Korean Neurosurg Soc. 2015; Dec. 58(6):518–27.5. Lee SH, Chung Y, Ryu JW, Choi SK, Kwun BD. Surgical revascularization for the treatment of complex anterior cerebral artery aneurysms: experience and illustrative review. World Neurosurg. 2018; Mar. 111:e507–18.6. Ota N, Tanikawa R, Miyama M, Matsumoto T, Miyazaki T, Matsukawa H, et al. Surgical strategy for complex anterior cerebral artery aneurysms: retrospective case series and literature review. World Neurosurg. 2016; Mar. 87:328–45.7. Sanai N, Zador Z, Lawton MT. Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass. Neurosurgery. 2009; Oct. 65(4):670–83; discussion 683.8. Spetzler RF, Roski RA, Rhodes RS, Modic MT. The “bonnet bypass”. Case report. J Neurosurg. 1980; Nov. 53(5):707–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Situ Rescue Bypass for Iatrogenic Avulsion of Parent Artery during Clipping Large Pericallosal Artery Aneurysm
- Surgical Treatment of Giant Serpentine Aneurysm of A2-A3 Segment Distal Anterior Cerebral Artery : Technical Case Report
- Stent-Assisted Coil Trapping in a Manual Internal Carotid Artery Compression Test for the Treatment of a Fusiform Dissecting Aneurysm
- In Situ Intersegmental Anastomosis within a Single Artery for Treatment of an Aneurysm at the Posterior Inferior Cerebellar Artery: Closing Omega Bypass
- A Giant Fusiform Aneurysm of Posterior Cerebral Artery Treated with Trapping after Temporal Lobectomy