J Korean Med Sci.
2023 Feb;38(8):e56. 10.3346/jkms.2023.38.e56.
Incidence and Economic Burden of Adverse Drug Reactions in Hospitalization: A Prospective Study in Korea
- Affiliations
-
- 1Division of Allergy and Clinical Immunology, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Pharmacovigilance Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Pulmonary, Allergy and Critical Care Medicine, Seongnam Citizens Medical Center, Seongnam, Korea
- 4Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 5Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 6Division of Allergy and Clinical Immunology, Department of Internal Medicine, Armed Forces Capital Hospital, Seongnam, Korea
- 7Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2539629
- DOI: http://doi.org/10.3346/jkms.2023.38.e56
Abstract
- Background
Adverse drug reactions (ADRs) are escalating, and their socioeconomic burden is increasing. However, large-scale prospective studies investigating ADRs during hospitalization are rare in Korea. We prospectively investigated the incidence, characteristics, and economic burden of ADRs in hospitalized patients based on electronic medical records (EMRs).
Methods
Among patients admitted to three hospitals from October 2016 to October 2017, 5,000 patients were randomly selected and prospectively observed during hospitalization. Research nurses monitored and detected patients who had symptoms, signs, or laboratory findings suspicious for ADRs using an EMR-based detection protocol. Next, allergy and ADR specialists reviewed the medical records to determine the relationship between adverse reactions and drugs. Cases in which a causal relationship was certain, probable/likely, or possible were included in the ADR cases. Clinically meaningful ADR cases or those leading to prolonged hospitalization were defined as significant ADRs.
Results
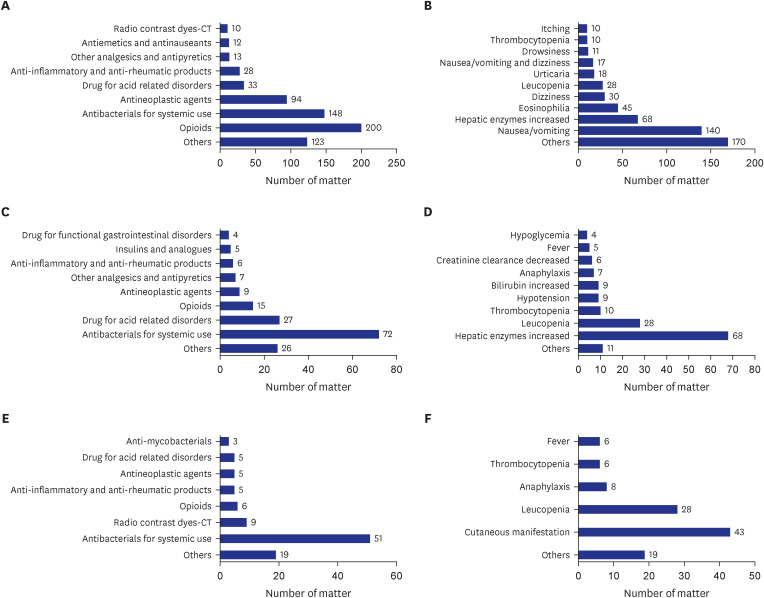
ADRs occurred in 510 (10.2%) patients. The mean length of hospital stay was approximately 5 days longer in patients with ADRs. Opioids accounted for the highest percentage of total ADRs. Significant ADRs were observed in 148 (3.0%) patients. Antibiotics accounted for the highest percentage of significant ADRs. Drug hypersensitivity reactions (DHRs) occurred in 88 (1.8%) patients. Antibiotics accounted for the highest percentage of DHRs. The average medical expenses for one day of hospitalization per patient were highest in significant ADRs, followed by non-significant ADRs, and non-ADRs.
Conclusion
ADRs in hospitalized patients are an important clinical issue, resulting in a substantial socioeconomic burden. EMR-based strategy could be a useful tool for ADR monitoring and early detection.
Figure
Reference
-
1. Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash). 2001; 41(2):192–199. PMID: 11297331.2. Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995; 155(18):1949–1956. PMID: 7575048.
Article3. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004; 329(7456):15–19. PMID: 15231615.4. van der Hooft CS, Dieleman JP, Siemes C, Aarnoudse AJ, Verhamme KM, Stricker BH, et al. Adverse drug reaction-related hospitalisations: a population-based cohort study. Pharmacoepidemiol Drug Saf. 2008; 17(4):365–371. PMID: 18302300.
Article5. Angamo MT, Chalmers L, Curtain CM, Bereznicki LR. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf. 2016; 39(9):847–857. PMID: 27449638.
Article6. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998; 279(15):1200–1205. PMID: 9555760.
Article7. Wester K, Jönsson AK, Spigset O, Druid H, Hägg S. Incidence of fatal adverse drug reactions: a population based study. Br J Clin Pharmacol. 2008; 65(4):573–579. PMID: 18070216.
Article8. Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One. 2009; 4(2):e4439. PMID: 19209224.
Article9. Kang DY, Ahn KM, Kang HR, Cho SH. Past, present, and future of pharmacovigilance in Korea. Asia Pac Allergy. 2017; 7(3):173–178. PMID: 28765823.
Article10. Molokhia M, Tanna S, Bell D. Improving reporting of adverse drug reactions: systematic review. Clin Epidemiol. 2009; 1:75–92. PMID: 20865089.
Article11. Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997; 277(4):301–306. PMID: 9002492.
Article12. Qing-ping S, Xiao-dong J, Feng D, Yan L, Mei-ling Y, Jin-xiu Z, et al. Consequences, measurement, and evaluation of the costs associated with adverse drug reactions among hospitalized patients in China. BMC Health Serv Res. 2014; 14(1):73. PMID: 24533894.13. Peter JV, Varghese GH, Alexander H, Tom NR, Swethalekshmi V, Truman C, et al. Patterns of adverse drug reaction in the medical wards of a teaching hospital: a prospective observational cohort study. Curr Drug Saf. 2016; 11(2):164–171. PMID: 26916785.
Article14. Giardina C, Cutroneo PM, Mocciaro E, Russo GT, Mandraffino G, Basile G, et al. Adverse drug reactions in hospitalized patients: results of the FORWARD (Facilitation of Reporting in Hospital Ward) study. Front Pharmacol. 2018; 9:350. PMID: 29695966.15. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000; 356(9237):1255–1259. PMID: 11072960.
Article16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40(5):373–383. PMID: 3558716.
Article17. Wolfe D, Yazdi F, Kanji S, Burry L, Beck A, Butler C, et al. Incidence, causes, and consequences of preventable adverse drug reactions occurring in inpatients: A systematic review of systematic reviews. PLoS One. 2018; 13(10):e0205426. PMID: 30308067.18. Fattahi F, Pourpak Z, Moin M, Kazemnejad A, Khotaei GT, Mamishi S, et al. Adverse drug reactions in hospitalized children in a department of infectious diseases. J Clin Pharmacol. 2005; 45(11):1313–1318. PMID: 16239365.19. Haffner S, von Laue N, Wirth S, Thürmann PA. Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf. 2005; 28(5):453–464. PMID: 15853446.20. Zopf Y, Rabe C, Neubert A, Hahn EG, Dormann H. Risk factors associated with adverse drug reactions following hospital admission: a prospective analysis of 907 patients in two German university hospitals. Drug Saf. 2008; 31(9):789–798. PMID: 18707193.
Article21. Bates DW, Miller EB, Cullen DJ, Burdick L, Williams L, Laird N, et al. Patient risk factors for adverse drug events in hospitalized patients. Arch Intern Med. 1999; 159(21):2553–2560. PMID: 10573045.
Article22. Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. JAMA. 1995; 274(1):29–34. PMID: 7791255.23. Bates DW, Spell N, Cullen DJ, Burdick E, Laird N, Petersen LA, et al. The costs of adverse drug events in hospitalized patients. JAMA. 1997; 277(4):307–311. PMID: 9002493.
Article24. Moore N, Lecointre D, Noblet C, Mabille M. Frequency and cost of serious adverse drug reactions in a department of general medicine. Br J Clin Pharmacol. 1998; 45(3):301–308. PMID: 9517375.
Article25. Thomas EJ, Brennan TA. Incidence and types of preventable adverse events in elderly patients: population based review of medical records. BMJ. 2000; 320(7237):741–744. PMID: 10720355.
Article26. Davies EC, Green CF, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a pilot study. J Clin Pharm Ther. 2006; 31(4):335–341. PMID: 16882102.27. Morimoto T, Sakuma M, Matsui K, Kuramoto N, Toshiro J, Murakami J, et al. Incidence of adverse drug events and medication errors in Japan: the JADE study. J Gen Intern Med. 2011; 26(2):148–153. PMID: 20872082.
Article28. Tian XY, Liu B, Shi H, Zhao ZR, Zhou XP, Zhang T, et al. Incidence of adverse cutaneous drug reactions in 22,866 Chinese inpatients: a prospective study. Arch Dermatol Res. 2015; 307(9):829–834. PMID: 26246330.29. Aljadhey H, Mahmoud MA, Ahmed Y, Sultana R, Zouein S, Alshanawani S, et al. Incidence of adverse drug events in public and private hospitals in Riyadh, Saudi Arabia: the (ADESA) prospective cohort study. BMJ Open. 2016; 6(7):e010831.30. Liao PJ, Mao CT, Chen TL, Deng ST, Hsu KH. Factors associated with adverse drug reaction occurrence and prognosis, and their economic impacts in older inpatients in Taiwan: a nested case-control study. BMJ Open. 2019; 9(5):e026771.
Article31. Pardo-Cabello AJ, Luna JD, Gómez Jiménez FJ, Del Pozo E, Puche Cañas E. Prevalence and risk factors associated with fatal adverse drug reactions among patients admitted at a Spanish teaching hospital. Eur J Intern Med. 2019; 70:e14–e16. PMID: 31630930.32. Aljadhey H, Mahmoud MA, Mayet A, Alshaikh M, Ahmed Y, Murray MD, et al. Incidence of adverse drug events in an academic hospital: a prospective cohort study. Int J Qual Health Care. 2013; 25(6):648–655. PMID: 24141014.
Article33. Geer MI, Koul PA, Tanki SA, Shah MY. Frequency, types, severity, preventability and costs of adverse drug reactions at a tertiary care hospital. J Pharmacol Toxicol Methods. 2016; 81:323–334. PMID: 27109493.
Article34. Kiguba R, Karamagi C, Bird SM. Incidence, risk factors and risk prediction of hospital-acquired suspected adverse drug reactions: a prospective cohort of Ugandan inpatients. BMJ Open. 2017; 7(1):e010568.
Article35. Suh DC, Woodall BS, Shin SK, Hermes-De Santis ER. Clinical and economic impact of adverse drug reactions in hospitalized patients. Ann Pharmacother. 2000; 34(12):1373–1379. PMID: 11144691.
Article36. Oderda GM, Said Q, Evans RS, Stoddard GJ, Lloyd J, Jackson K, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007; 41(3):400–406. PMID: 17341537.
Article37. Shamna M, Dilip C, Ajmal M, Linu Mohan P, Shinu C, Jafer CP, et al. A prospective study on adverse drug reactions of antibiotics in a tertiary care hospital. Saudi Pharm J. 2014; 22(4):303–308. PMID: 25161373.
Article38. Teschke R. Top-ranking drugs out of 3312 drug-induced liver injury cases evaluated by the Roussel Uclaf Causality Assessment Method. Expert Opin Drug Metab Toxicol. 2018; 14(11):1169–1187. PMID: 30354694.
Article39. Kang Y, Kim SH, Park SY, Park BY, Lee JH, An J, et al. Evaluation of drug-induced liver injury developed during hospitalization using electronic health record (EHR)-based algorithm. Allergy Asthma Immunol Res. 2020; 12(3):430–442. PMID: 32141257.
Article40. Clinton JW, Kiparizoska S, Aggarwal S, Woo S, Davis W, Lewis JH. Drug-induced liver injury: highlights and controversies in the recent literature. Drug Saf. 2021; 44(11):1125–1149. PMID: 34533782.
Article41. Kim SH. Active pharmacovigilance of drug-induced liver injury using electronic health records. Allergy Asthma Immunol Res. 2020; 12(3):378–380. PMID: 32141253.
Article42. Zeng Y, Dai Y, Zhou Z, Yu X, Shi D. Hepatotoxicity-related adverse effects of proton pump inhibitors: a cross-sectional study of signal mining and analysis of the FDA adverse event report system database. Front Med (Lausanne). 2021; 8:648164. PMID: 34869400.
Article43. Yu Z, Hu J, Hu Y. Neutropenia and thrombocytopenia induced by proton pump inhibitors: a case report. Drug Saf Case Rep. 2018; 5(1):28. PMID: 30470925.
Article44. Cohen S, Bueno de Mesquita M, Mimouni FB. Adverse effects reported in the use of gastroesophageal reflux disease treatments in children: a 10 years literature review. Br J Clin Pharmacol. 2015; 80(2):200–208. PMID: 25752807.
Article45. Thomson AB, Sauve MD, Kassam N, Kamitakahara H. Safety of the long-term use of proton pump inhibitors. World J Gastroenterol. 2010; 16(19):2323–2330. PMID: 20480516.
Article46. Gupta N, Patel C, Panda M. Hepatitis following famotidine: a case report. Cases J. 2009; 2(1):89. PMID: 19173722.
Article47. Gomes ER, Demoly P. Epidemiology of hypersensitivity drug reactions. Curr Opin Allergy Clin Immunol. 2005; 5(4):309–316. PMID: 15985812.
Article48. Wong A, Seger DL, Lai KH, Goss FR, Blumenthal KG, Zhou L. Drug hypersensitivity reactions documented in electronic health records within a large health system. J Allergy Clin Immunol Pract. 2019; 7(4):1253–1260.e3. PMID: 30513361.
Article49. Ahmad J, Odin JA. Epidemiology and genetic risk factors of drug hepatotoxicity. Clin Liver Dis. 2017; 21(1):55–72. PMID: 27842775.
Article50. Saff RR, Li Y, Santhanakrishnan N, Camargo CA Jr, Blumenthal KG, Zhou L, et al. Identification of inpatient allergic drug reactions using ICD-9-CM codes. J Allergy Clin Immunol Pract. 2019; 7(1):259–264.e1. PMID: 30075337.
Article51. Park CS, Kim TB, Kim SL, Kim JY, Yang KA, Bae YJ, et al. The use of an electronic medical record system for mandatory reporting of drug hypersensitivity reactions has been shown to improve the management of patients in the university hospital in Korea. Pharmacoepidemiol Drug Saf. 2008; 17(9):919–925. PMID: 18470952.
Article52. Klein LE, German PS, Levine DM. Adverse drug reactions among the elderly: a reassessment. J Am Geriatr Soc. 1981; 29(11):525–530. PMID: 7028845.
Article53. Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007; 30(5):379–407. PMID: 17472418.54. Brahma DK, Wahlang JB, Marak MD, Ch Sangma M. Adverse drug reactions in the elderly. J Pharmacol Pharmacother. 2013; 4(2):91–94. PMID: 23761706.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse Drug Reactions to Dapsone: A Retrospective Study during the Last 15 Years
- The frequency of adverse drug reactions in a tertiary care hostpital in Korea
- Social Burden of Drug Allergy and its Prevention
- Relief System for Adverse Drug Reactions in Korea
- Active Participation in Reporting Adverse Drug Reactions