J Korean Med Sci.
2023 Feb;38(5):e33. 10.3346/jkms.2023.38.e33.
Nationwide Treatment Outcomes of Patients With Multidrug/RifampinResistant Tuberculosis in Korea, 2011–2017: A Retrospective Cohort Study (Korean TB-POST)
- Affiliations
-
- 1Department of Preventive Medicine, Konyang University College of Medicine, Daejeon, Korea
- 2Department of Internal Medicine, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea
- 3Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Research and Development Center, the Korean Institute of Tuberculosis, Korean National Tuberculosis Association, Cheongju, Korea
- 5Department of Health Policy and Management, Seoul National University College of Medicine, Seoul, Korea
- 6Central Training Institute, Korean National Tuberculosis Association, Seoul, Korea
- 7Department of Health Policy Research, National Evidence-Based Healthcare Collaborating Agency, Seoul, Korea
- 8Department of Internal Medicine, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- KMID: 2539197
- DOI: http://doi.org/10.3346/jkms.2023.38.e33
Abstract
- Background
The treatment outcomes of patients with multidrug/rifampin-resistant (MDR/RR) tuberculosis (TB) are important indicators that reflect the current status of TB management and identify the key challenges encountered by TB control programs in a country.
Methods
We retrospectively evaluated the treatment outcomes as well as predictors of unfavorable outcomes in patients with MDR/RR-TB notified from 2011 to 2017, using an integrated TB database.
Results
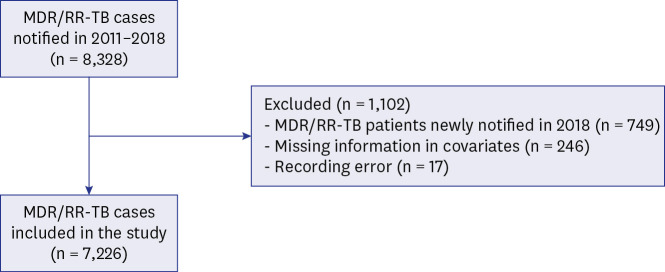
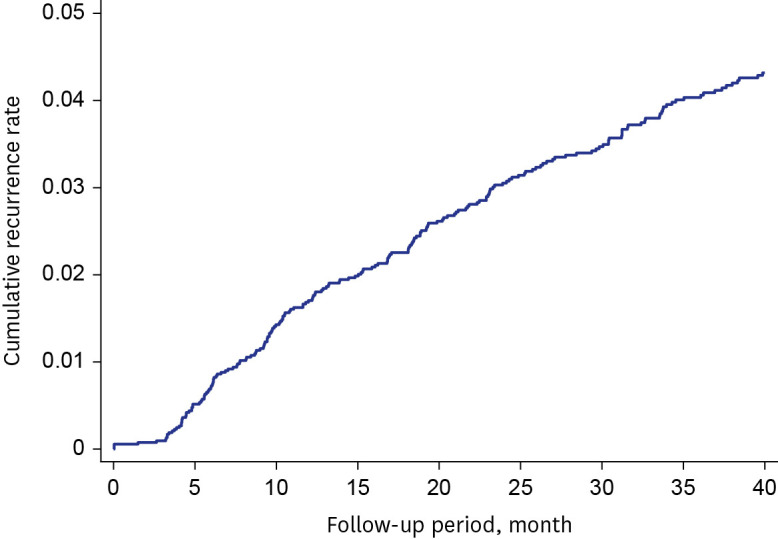
A total of 7,226 patients with MDR/RR-TB were included. The treatment success rate had significantly increased from 63.9% in 2011 to 75.1% in 2017 (P < 0.001). Among unfavorable outcomes, the proportion of patients who failed, were lost to follow up, and were not evaluated had gradually decreased (P< 0.001). In contrast, TB-related death rate was not significantly changed (P= 0.513), while the non-TB related death rate had increased from 3.2% in 2011 to 11.1% in 2017 (P < 0.001). Older age, male sex, immigrants, low household income, previous history of TB treatment, and comorbidities were independent predictors of unfavorable outcomes. Of the 5,308 patients who were successfully treated, recurrence occurred in 241 patients (4.5%) at a median 18.4 months (interquartile range, 9.2–32.4) after completion treatment.
Conclusion
The treatment outcomes of patients with MDR/RR-TB has gradually improved but increasing deaths during treatment is an emerging challenge for MDR-TB control in Korea. Targeted and comprehensive care is needed for vulnerable patients such as the elderly, patients with comorbidities, and those with low household incomes.
Figure
Reference
-
1. Dheda K, Gumbo T, Maartens G, Dooley KE, Murray M, Furin J, et al. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med. 2019; 7(9):820–826. PMID: 31486393.2. Korea Disease Control and Prevention Agency. Annual report on the notified tuberculosis patients in Korea 2021. Updated 2022. Accessed June 1, 2022. https://www.kdca.go.kr/npt/biz/npp/portal/nppPblctDtaView.do?pblctDtaSeAt=1&pblctDtaSn=2598 .3. Kwak N, Kim HR, Yoo CG, Kim YW, Han SK, Yim JJ. Multidrug-resistant tuberculosis over 20 years at a referral hospital in South Korea: trends and outcomes. Int J Tuberc Lung Dis. 2019; 23(2):174–180. PMID: 30808449.4. Lee EH, Yong SH, Leem AY, Lee SH, Kim SY, Chung KS, et al. Improved fluoroquinolone-resistant and extensively drug-resistant tuberculosis treatment outcomes. Open Forum Infect Dis. 2019; 6(4):ofz118. PMID: 30949546.5. Kang Y, Jo EJ, Eom JS, Kim MH, Lee K, Kim KU, et al. Treatment outcomes of patients with multidrug-resistant tuberculosis: comparison of pre- and post-public-private mix periods. Tuberc Respir Dis (Seoul). 2021; 84(1):74–83. PMID: 33108860.6. Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008; 178(10):1075–1082. PMID: 18703792.7. Kang YA, Shim TS, Koh WJ, Lee SH, Lee CH, Choi JC, et al. Choice between levofloxacin and moxifloxacin and multidrug-resistant tuberculosis treatment outcomes. Ann Am Thorac Soc. 2016; 13(3):364–370. PMID: 26871879.8. Hwang H, Kang H, Kwon YS, Jeon D, Shim TS, Yim JJ. Outcomes of multidrug-resistant tuberculosis treated with bedaquiline or delamanid. Clin Infect Dis. 2021; 73(8):1362–1369. PMID: 33837767.9. Lee M, Han J, Kim YR, Kwak N, Kim JH, Park O, et al. Multidrug-resistant tuberculosis in South Korea: a retrospective analysis of national registry data in 2011–2015. Int J Tuberc Lung Dis. 2019; 23(7):850–857. PMID: 31439118.10. Yoo JE, Kim D, Han K, Rhee SY, Shin DW, Lee H. Diabetes status and association with risk of tuberculosis among Korean adults. JAMA Netw Open. 2021; 4(9):e2126099. PMID: 34546370.11. Min J, Kim HW, Koo HK, Ko Y, Oh JY, Kim J, et al. Impact of COVID-19 pandemic on the national PPM tuberculosis control project in Korea: the Korean PPM monitoring database between July 2019 and June 2020. J Korean Med Sci. 2020; 35(43):e388. PMID: 33169559.12. Kim HW, Min J, Choi JY, Shin AY, Myong JP, Lee Y, et al. Latent tuberculosis infection screening and treatment in congregate settings (TB FREE COREA): demographic profiles of interferon-gamma release assay cohort. J Korean Med Sci. 2021; 36(36):e246. PMID: 34519187.13. Min J, Kim HW, Choi JY, Shin AY, Kang JY, Lee Y, et al. Latent tuberculosis cascade of care among healthcare workers: a nationwide cohort analysis in Korea between 2017 and 2018. J Korean Med Sci. 2022; 37(20):e164. PMID: 35607742.14. Jeong D, Kang HY, Kim J, Lee H, Yoo BN, Kim HS, et al. Cohort profile: Korean tuberculosis and post-tuberculosis cohort constructed by linking the Korean National Tuberculosis Surveillance System and National Health Information Database. J Prev Med Public Health. 2022; 55(3):253–262. PMID: 35677999.15. World Health Organization. Definitions and reporting framework for tuberculosis - 2013 revision. Updated 2020. Accessed June 1, 2022. https://apps.who.int/iris/bitstream/handle/10665/79199/9789241505345_eng.pdf?sequence=1 .16. Gill CM, Dolan L, Piggott LM, McLaughlin AM. New developments in tuberculosis diagnosis and treatment. Breathe (Sheff). 2022; 18(1):210149. PMID: 35284018.17. Jeon D, Kang H, Kwon YS, Yim JJ, Shim TS. Impact of molecular drug susceptibility testing on the time to multidrug-resistant tuberculosis treatment initiation. J Korean Med Sci. 2020; 35(35):e284. PMID: 32893517.18. Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JC, Anderson LF, Baghaei P, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018; 392(10150):821–834. PMID: 30215381.19. Schnippel K, Ndjeka N, Maartens G, Meintjes G, Master I, Ismail N, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: a retrospective cohort study. Lancet Respir Med. 2018; 6(9):699–706. PMID: 30001994.20. Ndjeka N, Campbell JR, Meintjes G, Maartens G, Schaaf HS, Hughes J, et al. Treatment outcomes 24 months after initiating short, all-oral bedaquiline-containing or injectable-containing rifampicin-resistant tuberculosis treatment regimens in South Africa: a retrospective cohort study. Lancet Infect Dis. 2022; 22(7):1042–1051. PMID: 35512718.21. Son E, Jeon D. Current situation of tuberculosis and National Strategic Plan for Tuberculosis Control in Korea. J Korean Med Assoc. 2021; 64(4):316–323.22. Negin J, Abimbola S, Marais BJ. Tuberculosis among older adults--time to take notice. Int J Infect Dis. 2015; 32:135–137. PMID: 25809769.23. Byng-Maddick R, Noursadeghi M. Does tuberculosis threaten our ageing populations? BMC Infect Dis. 2016; 16(1):119. PMID: 26968654.24. Duarte R, Lönnroth K, Carvalho C, Lima F, Carvalho AC, Muñoz-Torrico M, et al. Tuberculosis, social determinants and co-morbidities (including HIV). Pulmonology. 2018; 24(2):115–119. PMID: 29275968.25. Creswell J, Raviglione M, Ottmani S, Migliori GB, Uplekar M, Blanc L, et al. Tuberculosis and noncommunicable diseases: neglected links and missed opportunities. Eur Respir J. 2011; 37(5):1269–1282. PMID: 20947679.26. Lönnroth K, Glaziou P, Weil D, Floyd K, Uplekar M, Raviglione M. Beyond UHC: monitoring health and social protection coverage in the context of tuberculosis care and prevention. PLoS Med. 2014; 11(9):e1001693. PMID: 25243782.27. Jeong I, Kim HJ, Kim J, Oh SY, Lee JB, Bai JY, et al. Diagnostic accuracy of notified cases as pulmonary tuberculosis in private sectors of Korea. J Korean Med Sci. 2012; 27(5):525–531. PMID: 22563218.28. Kang HY, Yoo H, Park W, Go U, Jeong E, Jung KS, et al. Tuberculosis notification completeness and timeliness in the Republic of Korea during 2012–2014. Osong Public Health Res Perspect. 2016; 7(5):320–326. PMID: 27812491.29. Min J, Kim HW, Ko Y, Oh JY, Kang JY, Lee J, et al. Tuberculosis surveillance and monitoring under the national public-private mix tuberculosis control project in South Korea 2016–2017. Tuberc Respir Dis (Seoul). 2020; 83(3):218–227.30. Jang SJ, Hwang MJ, Lee CH, Lee HJ, Shim TS, Kim DS. Change in quality of tuberculosis (TB) care since national quality assessment program of TB healthcare service. Qual Improv Health Care. 2021; 27(2):73–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Anti-Tuberculosis Drug Use on Treatment Outcomes in Patients with Pulmonary Fluoroquinolone-Resistant Multidrug-Resistant Tuberculosis: A Nationwide Retrospective Cohort Study with Propensity Score Matching
- Issues Related to the Updated 2014 Korean Guidelines for Tuberculosis
- WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update: Applicability in South Korea
- Treatment Outcomes of Patients with Multidrug-Resistant Tuberculosis: Comparison of Pre- and Post-Public–Private Mix Periods
- Clinical Implications of New Drugs and Regimens for the Treatment of Drug-resistant Tuberculosis