J Stroke.
2023 Jan;25(1):39-54. 10.5853/jos.2022.02173.
The Heart Is at Risk: Understanding Stroke-Heart-Brain Interactions with Focus on Neurogenic Stress Cardiomyopathy—A Review
- Affiliations
-
- 1Department of Internal Medicine, Thun General Hospital, Thun, Switzerland
- 2Department of Emergency Medicine, Inselspital, University Hospital, University of Bern, Bern, Switzerland
- KMID: 2539057
- DOI: http://doi.org/10.5853/jos.2022.02173
Abstract
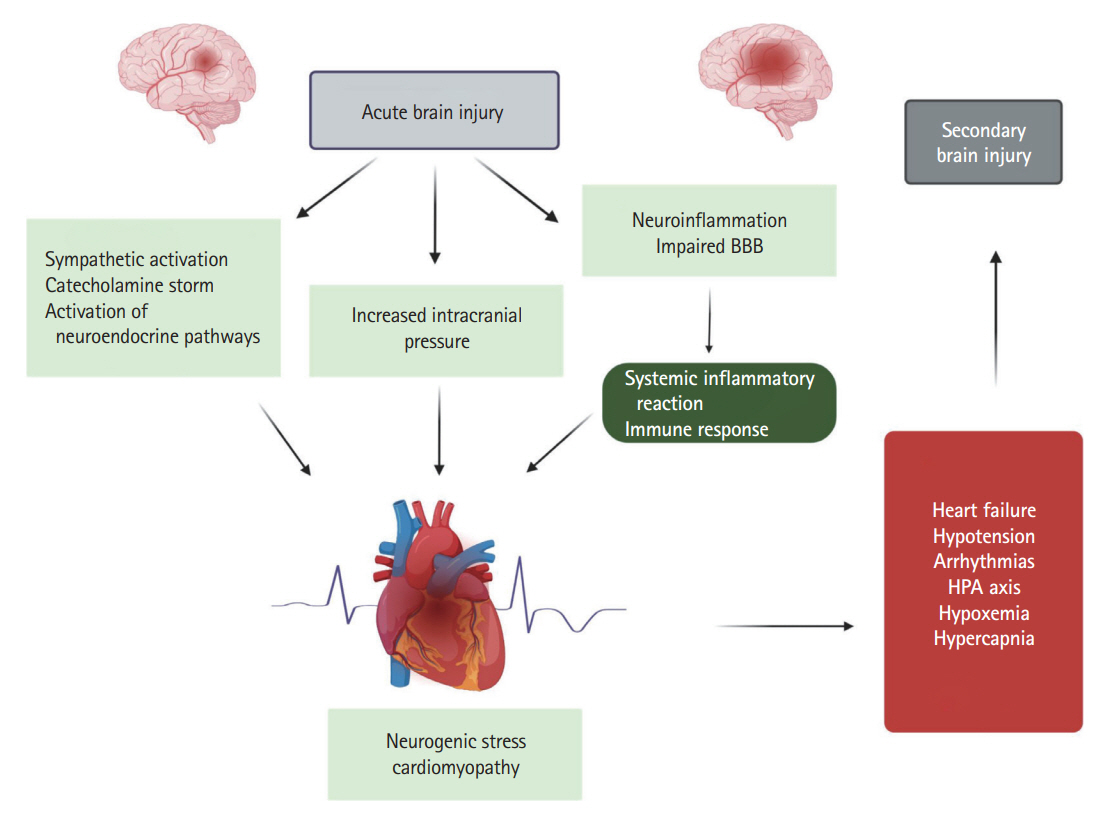
- In recent years, it has been convincingly demonstrated that acute brain injury may cause severe cardiac complications—such as neurogenic stress cardiomyopathy (NSC), a specific form of takotsubo cardiomyopathy. The pathophysiology of these brain-heart interactions is complex and involves sympathetic hyperactivity, activation of the hypothalamic-pituitary-adrenal axis, as well as immune and inflammatory pathways. There have been great strides in our understanding of the axis from the brain to the heart in patients with isolated acute brain injury and more specifically in patients with stroke. On the other hand, in patients with NSC, research has mainly focused on hemodynamic dysfunction due to arrhythmias, regional wall motion abnormality, or left ventricular hypokinesia that leads to impaired cerebral perfusion pressure. Comparatively little is known about the underlying secondary and delayed cerebral complications. The aim of the present review is to describe the stroke-heart-brain axis and highlight the main pathophysiological mechanisms leading to secondary and delayed cerebral injury in patients with concurrent hemorrhagic or ischemic stroke and NSC as well as to identify further areas of research that could potentially improve outcomes in this specific patient population.
Keyword
Figure
Reference
-
References
1. Mrozek S, Gobin J, Constantin JM, Fourcade O, Geeraerts T. Crosstalk between brain, lung and heart in critical care. Anaesth Crit Care Pain Med. 2020; 39:519–530.2. Gopinath R, Ayya SS. Neurogenic stress cardiomyopathy: what do we need to know. Ann Card Anaesth. 2018; 21:228–234.3. Chen Z, Venkat P, Seyfried D, Chopp M, Yan T, Chen J. Brainheart interaction: cardiac complications after stroke. Circ Res. 2017; 121:451–468.4. Biso S, Wongrakpanich S, Agrawal A, Yadlapati S, Kishlyansky M, Figueredo V. A review of neurogenic stunned myocardium. Cardiovasc Psychiatry Neurol. 2017; 2017:5842182.5. Lee VH, Oh JK, Mulvagh SL, Wijdicks EF. Mechanisms in neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2006; 5:243–249.6. Robba C, Bonatti G, Pelosi P, Citerio G. Extracranial complications after traumatic brain injury: targeting the brain and the body. Curr Opin Crit Care. 2020; 26:137–146.7. Dantzer R, Konsman JP, Bluthé RM, Kelley KW. Neural and humoral pathways of communication from the immune system to the brain: parallel or convergent? Auton Neurosci. 2000; 85:60–65.8. Kerro A, Woods T, Chang JJ. Neurogenic stunned myocardium in subarachnoid hemorrhage. J Crit Care. 2017; 38:27–34.9. Krishnamoorthy V, Mackensen GB, Gibbons EF, Vavilala MS. Cardiac dysfunction after neurologic injury: what do we know and where are we going? Chest. 2016; 149:1325–1331.10. Havakuk O, King KS, Grazette L, Yoon AJ, Fong M, Bregman N, et al. Heart failure-induced brain injury. J Am Coll Cardiol. 2017; 69:1609–1616.11. Claassen JAHR, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021; 101:1487–1559.12. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017; 60:1–12.13. Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015; 12:114.14. Ziaka M, Exadaktylos A. Brain-lung interactions and mechanical ventilation in patients with isolated brain injury. Crit Care. 2021; 25:358.15. de Gregorio C. Cardioembolic outcomes in stress-related cardiomyopathy complicated by ventricular thrombus: a systematic review of 26 clinical studies. Int J Cardiol. 2010; 141:11–17.16. Kenigsberg BB, Barnett CF, Mai JC, Chang JJ. Neurogenic stunned myocardium in severe neurological injury. Curr Neurol Neurosci Rep. 2019; 19:90.17. Porto I, Della Bona R, Leo A, Proietti R, Pieroni M, Caltagirone C, et al. Stress cardiomyopathy (tako-tsubo) triggered by nervous system diseases: a systematic review of the reported cases. Int J Cardiol. 2013; 167:2441–2448.18. Sposato LA, Fridman S, Whitehead SN, Lopes RD. Linking stroke-induced heart injury and neurogenic atrial fibrillation: a hypothesis to be proven. J Electrocardiol. 2018; 51:430–432.19. Sposato LA, Hilz MJ, Aspberg S, Murthy SB, Bahit MC, Hsieh CY, et al. Post-stroke cardiovascular complications and neurogenic cardiac injury: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 76:2768–2785.20. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015; 373:929–938.21. Scheitz JF, Nolte CH, Doehner W, Hachinski V, Endres M. Stroke-heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol. 2018; 17:1109–1120.22. Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of takotsubo syndrome. Circulation. 2017; 135:2426–2441.23. Balint B, Jaremek V, Thorburn V, Whitehead SN, Sposato LA. Left atrial microvascular endothelial dysfunction, myocardial inflammation and fibrosis after selective insular cortex ischemic stroke. Int J Cardiol. 2019; 292:148–155.24. Suzuki H, Matsumoto Y, Kaneta T, Sugimura K, Takahashi J, Fukumoto Y, et al. Evidence for brain activation in patients with takotsubo cardiomyopathy. Circ J. 2014; 78:256–258.25. Masuda T, Sato K, Yamamoto S, Matsuyama N, Shimohama T, Matsunaga A, et al. Sympathetic nervous activity and myocardial damage immediately after subarachnoid hemorrhage in a unique animal model. Stroke. 2002; 33:1671–1676.26. Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S; VISTA Investigators. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007; 38:2295–2302.27. Kallmünzer B, Breuer L, Kahl N, Bobinger T, Raaz-Schrauder D, Huttner HB, et al. Serious cardiac arrhythmias after stroke: incidence, time course, and predictors--a systematic, prospective analysis. Stroke. 2012; 43:2892–2897.28. Jensen JK, Ueland T, Aukrust P, Antonsen L, Kristensen SR, Januzzi JL, et al. Highly sensitive troponin T in patients with acute ischemic stroke. Eur Neurol. 2012; 68:287–293.29. Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008; 5:22–29.30. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International expert consensus document on takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018; 39:2032–2046.31. White M, Wiechmann RJ, Roden RL, Hagan MB, Wollmering MM, Port JD, et al. Cardiac beta-adrenergic neuroeffector systems in acute myocardial dysfunction related to brain injury. Evidence for catecholamine-mediated myocardial damage. Circulation. 1995; 92:2183–2189.32. Paur H, Wright PT, Sikkel MB, Tranter MH, Mansfield C, O’Gara P, et al. High levels of circulating epinephrine trigger apical cardiodepression in a β2-adrenergic receptor/Gi-dependent manner: a new model of takotsubo cardiomyopathy. Circulation. 2012; 126:697–706.33. Jung JM, Kim JG, Kim JB, Cho KH, Yu S, Oh K, et al. Takotsubo-like myocardial dysfunction in ischemic stroke: a hospital-based registry and systematic literature review. Stroke. 2016; 47:2729–2736.34. Yoshimura S, Toyoda K, Ohara T, Nagasawa H, Ohtani N, Kuwashiro T, et al. Takotsubo cardiomyopathy in acute ischemic stroke. Ann Neurol. 2008; 64:547–554.35. Bleilevens C, Roehl AB, Zoremba N, Tolba R, Rossaint R, Hein M. Insular infarct size but not levosimendan influenced myocardial injury triggered by cerebral ischemia in rats. Exp Brain Res. 2015; 233:149–156.36. Nagai M, Hoshide S, Kario K. The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J Am Soc Hypertens. 2010; 4:174–182.37. Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009; 10:59–70.38. Mashaly HA, Provencio JJ. Inflammation as a link between brain injury and heart damage: the model of subarachnoid hemorrhage. Cleve Clin J Med. 2008; 75 Suppl 2:S26–S30.39. Wang X, Cui L, Ji X. Cognitive impairment caused by hypoxia: from clinical evidences to molecular mechanisms. Metab Brain Dis. 2022; 37:51–66.40. Tavazzi G, Zanierato M, Via G, Iotti GA, Procaccio F. Are neurogenic stress cardiomyopathy and takotsubo different syndromes with common pathways?: etiopathological insights on dysfunctional hearts. JACC Heart Fail. 2017; 5:940–942.41. Fassbender K, Schmidt R, Mössner R, Daffertshofer M, Hennerici M. Pattern of activation of the hypothalamic-pituitary-adrenal axis in acute stroke. Relation to acute confusional state, extent of brain damage, and clinical outcome. Stroke. 1994; 25:1105–1108.42. Sobowale OA, Parry-Jones AR, Smith CJ, Tyrrell PJ, Rothwell NJ, Allan SM. Interleukin-1 in stroke: from bench to bedside. Stroke. 2016; 47:2160–2167.43. Edwards AV, Jones CT. Autonomic control of adrenal function. J Anat. 1993; 183(Pt 2):291–307.44. Battaglini D, Robba C, Lopes da Silva A, Dos Santos Samary C, Leme Silva P, Dal Pizzol F, et al. Brain-heart interaction after acute ischemic stroke. Crit Care. 2020; 24:163.45. Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005; 352:539–548.46. Kono T, Morita H, Kuroiwa T, Onaka H, Takatsuka H, Fujiwara A. Left ventricular wall motion abnormalities in patients with subarachnoid hemorrhage: neurogenic stunned myocardium. J Am Coll Cardiol. 1994; 24:636–640.47. Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. XIV. Hyperventilation. J Neurotrauma. 2007; 24 Suppl 1:S87–S90.48. Nguyen H, Zaroff JG. Neurogenic stunned myocardium. Curr Neurol Neurosci Rep. 2009; 9:486–491.49. Mazzeo AT, Micalizzi A, Mascia L, Scicolone A, Siracusano L. Brain-heart crosstalk: the many faces of stress-related cardiomyopathy syndromes in anaesthesia and intensive care. Br J Anaesth. 2014; 112:803–815.50. Banki NM, Kopelnik A, Dae MW, Miss J, Tung P, Lawton MT, et al. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation. 2005; 112:3314–3319.51. Sugimoto K, Watanabe E, Yamada A, Iwase M, Sano H, Hishida H, et al. Prognostic implications of left ventricular wall motion abnormalities associated with subarachnoid hemorrhage. Int Heart J. 2008; 49:75–85.52. Malik AN, Gross BA, Rosalind Lai PM, Moses ZB, Du R. Neurogenic stress cardiomyopathy after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2015; 83:880–885.53. Kothavale A, Banki NM, Kopelnik A, Yarlagadda S, Lawton MT, Ko N, et al. Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocrit Care. 2006; 4:199–205.54. Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, et al. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004; 35:548–551.55. Kilbourn KJ, Levy S, Staff I, Kureshi I, McCullough L. Clinical characteristics and outcomes of neurogenic stress cadiomyopathy in aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg. 2013; 115:909–914.56. Colivicchi F, Bassi A, Santini M, Caltagirone C. Cardiac autonomic derangement and arrhythmias in right-sided stroke with insular involvement. Stroke. 2004; 35:2094–2098.57. Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM. Left insular stroke is associated with adverse cardiac outcome. Neurology. 2006; 66:477–483.58. Krause T, Werner K, Fiebach JB, Villringer K, Piper SK, Haeusler KG, et al. Stroke in right dorsal anterior insular cortex is related to myocardial injury. Ann Neurol. 2017; 81:502–511.59. Cheung RT, Hachinski V. Cardiac effects of stroke. Curr Treat Options Cardiovasc Med. 2004; 6:199–207.60. Oras J, Grivans C, Bartley A, Rydenhag B, Ricksten SE, Seeman-Lodding H. Elevated high-sensitive troponin T on admission is an indicator of poor long-term outcome in patients with subarachnoid haemorrhage: a prospective observational study. Crit Care. 2016; 20:11.61. Murthy SB, Shah S, Rao CP, Bershad EM, Suarez JI. Neurogenic stunned myocardium following acute subarachnoid hemorrhage: pathophysiology and practical considerations. J Intensive Care Med. 2015; 30:318–325.62. Nasr DM, Tomasini S, Prasad A, Rabinstein AA. Acute brain diseases as triggers for stress cardiomyopathy: clinical characteristics and outcomes. Neurocrit Care. 2017; 27:356–361.63. Mihalovic M, Tousek P. Myocardial injury after stroke. J Clin Med. 2022; 11:2.64. Scheitz JF, Stengl H, Nolte CH, Landmesser U, Endres M. Neurological update: use of cardiac troponin in patients with stroke. J Neurol. 2021; 268:2284–2292.65. Alkhachroum AM, Miller B, Chami T, Tatsuoka C, Sila C. A troponin study on patients with ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage: type II myocardial infarction is significantly associated with stroke severity, discharge disposition and mortality. J Clin Neurosci. 2019; 64:83–88.66. Hravnak M, Frangiskakis JM, Crago EA, Chang Y, Tanabe M, Gorcsan J 3rd, et al. Elevated cardiac troponin I and relationship to persistence of electrocardiographic and echocardiographic abnormalities after aneurysmal subarachnoid hemorrhage. Stroke. 2009; 40:3478–3484.67. Christensen H, Fogh Christensen A, Boysen G. Abnormalities on ECG and telemetry predict stroke outcome at 3 months. J Neurol Sci. 2005; 234:99–103.68. Iltumur K, Yavavli A, Apak I, Ariturk Z, Toprak N. Elevated plasma N-terminal pro-brain natriuretic peptide levels in acute ischemic stroke. Am Heart J. 2006; 151:1115–1122.69. Montaner J, García-Berrocoso T, Mendioroz M, Palacios M, Perea-Gainza M, Delgado P, et al. Brain natriuretic peptide is associated with worsening and mortality in acute stroke patients but adds no prognostic value to clinical predictors of outcome. Cerebrovasc Dis. 2012; 34:240–245.70. Sviri GE, Soustiel JF, Zaaroor M. Alteration in brain natriuretic peptide (BNP) plasma concentration following severe traumatic brain injury. Acta Neurochir (Wien). 2006; 148:529–533. discussion 533.71. Etgen T, Baum H, Sander K, Sander D. Cardiac troponins and N-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke. 2005; 36:270–275.72. Takahashi K, Totsune K, Sone M, Ohneda M, Murakami O, Itoi K, et al. Human brain natriuretic peptide-like immunoreactivity in human brain. Peptides. 1992; 13:121–123.73. Park KI. Plasma brain-type natriuretic peptide level following seizure and syncope: pilot study. J Epilepsy Res. 2014; 4:14–17.74. Agewall S, Giannitsis E, Jernberg T, Katus H. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2011; 32:404–411.75. Wira CR 3rd, Rivers E, Martinez-Capolino C, Silver B, Iyer G, Sherwin R, et al. Cardiac complications in acute ischemic stroke. West J Emerg Med. 2011; 12:414–420.76. Krishnamoorthy V, Rowhani-Rahbar A, Gibbons EF, Rivara FP, Temkin NR, Pontius C, et al. Early systolic dysfunction following traumatic brain injury: a cohort study. Crit Care Med. 2017; 45:1028–1036.77. Hüttemann E, Schelenz C, Chatzinikolaou K, Reinhart K. Left ventricular dysfunction in lethal severe brain injury: impact of transesophageal echocardiography on patient management. Intensive Care Med. 2002; 28:1084–1088.78. Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017; 19:821–836.79. Wang X, Pei J, Hu X. The brain-heart connection in takotsubo syndrome: the central nervous system, sympathetic nervous system, and catecholamine overload. Cardiol Res Pract. 2020; 9. 2020:4150291.80. Klein C, Hiestand T, Ghadri JR, Templin C, Jäncke L, Hänggi J. Takotsubo syndrome - predictable from brain imaging data. Sci Rep. 2017; 7:5434.81. Templin C, Hänggi J, Klein C, Topka MS, Hiestand T, Levinson RA, et al. Altered limbic and autonomic processing supports brain-heart axis in takotsubo syndrome. Eur Heart J. 2019; 40:1183–1187.82. Hiestand T, Hänggi J, Klein C, Topka MS, Jaguszewski M, Ghadri JR, et al. Takotsubo syndrome associated with structural brain alterations of the limbic system. J Am Coll Cardiol. 2018; 7:809–811.83. Kewcharoen J, Trongtorsak A, Kanitsoraphan C, Prasitlumkum N, Mekritthikrai R, Techorueangwiwat C, et al. Cognitive impairment and 30-day rehospitalization rate in patients with acute heart failure: a systematic review and meta-analysis. Indian Heart J. 2019; 71:52–59.84. Mccarthy ST, Wollner L, Rosenberg GA, Haaland KY. Cardiogenic dementia. Lancet. 1981; 318:1171.85. Zuccalà G, Onder G, Pedone C, Carosella L, Pahor M, Bernabei R, et al. Hypotension and cognitive impairment: selective association in patients with heart failure. Neurology. 2001; 57:1986–1992.86. Georgiadis D, Schwarz S, Baumgartner RW, Veltkamp R, Schwab S. Influence of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure in patients with acute stroke. Stroke. 2001; 32:2088–2092.87. Ziaka M, Exadaktylos A. ARDS associated acute brain injury: from the lung to the brain. Eur J Med Res. 2022; 27:150.88. Kim SM, Aikat S, Bailey A, White M. Takotsubo cardiomyopathy as a source of cardioembolic cerebral infarction. BMJ Case Rep. 2012; 2012:bcr2012006835.89. Phillips AA, Chan FH, Zheng MM, Krassioukov AV, Ainslie PN. Neurovascular coupling in humans: physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab. 2016; 36:647–664.90. Willie CK, Smith KJ. Fuelling the exercising brain: a regulatory quagmire for lactate metabolism. J Physiol. 2011; 589(Pt 4):779–780.91. Paulson OB, Jarden JO, Godtfredsen J, Vorstrup S. Cerebral blood flow in patients with congestive heart failure treated with captopril. Am J Med. 1984; 76:91–95.92. Choi BR, Kim JS, Yang YJ, Park KM, Lee CW, Kim YH, et al. Factors associated with decreased cerebral blood flow in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 2006; 97:1365–1369.93. Jefferson AL, Himali JJ, Au R, Seshadri S, Decarli C, O’Donnell CJ, et al. Relation of left ventricular ejection fraction to cognitive aging (from the Framingham Heart Study). Am J Cardiol. 2011; 108:1346–1351.94. de la Torre JC. Critically attained threshold of cerebral hypoperfusion: the CATCH hypothesis of Alzheimer’s pathogenesis. Neurobiol Aging. 2000; 21:331–342.95. Xu C, Tao X, Ma X, Zhao R, Cao Z. Cognitive dysfunction after heart disease: a manifestation of the heart-brain axis. Oxid Med Cell Longev. 2021; 2021:4899688.96. Tini G, Scagliola R, Monacelli F, La Malfa G, Porto I, Brunelli C, et al. Alzheimer’s disease and cardiovascular disease: a particular association. Cardiol Res Pract. 2020; 2020:2617970.97. Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. 2016; 34:465–477.98. Griesdale DE, Örtenwall V, Norena M, Wong H, Sekhon MS, Kolmodin L, et al. Adherence to guidelines for management of cerebral perfusion pressure and outcome in patients who have severe traumatic brain injury. J Crit Care. 2015; 30:111–115.99. Prabhakar H, Sandhu K, Bhagat H, Durga P, Chawla R. Current concepts of optimal cerebral perfusion pressure in traumatic brain injury. J Anaesthesiol Clin Pharmacol. 2014; 30:318–327.100. Czosnyka M, Smielewski P, Piechnik S, Steiner LA, Pickard JD. Cerebral autoregulation following head injury. J Neurosurg. 2001; 95:756–763.101. Hoiland RL, Bain AR, Rieger MG, Bailey DM, Ainslie PN. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. Am J Physiol Regul Integr Comp Physiol. 2016; 310:R398–R413.102. Ziaka M, Makris D, Fotakopoulos G, Tsilioni I, Befani C, Liakos P, et al. High-tidal-volume mechanical ventilation and lung inflammation in intensive care patients with normal lungs. Am J Crit Care. 2020; 29:15–21.103. Derdeyn CP, Videen TO, Yundt KD, Fritsch SM, Carpenter DA, Grubb RL, et al. Variability of cerebral blood volume and oxygen extraction: stages of cerebral haemodynamic impairment revisited. Brain. 2002; 125(Pt 3):595–607.104. Lorenzi-Filho G, Azevedo ER, Parker JD, Bradley TD. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002; 19:37–40.105. Thompson BT. Glucocorticoids and acute lung injury. Crit Care Med. 2003; 31(4 Suppl):S253–S257.106. Hill AR, Spencer-Segal JL. Glucocorticoids and the brain after critical illness. Endocrinology. 2021; 162:bqaa242.107. Güder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, et al. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007; 115:1754–1761.108. Huffman JC, Celano CM, Beach SR, Motiwala SR, Januzzi JL. Depression and cardiac disease: epidemiology, mechanisms, and diagnosis. Cardiovasc Psychiatry Neurol. 2013; 2013:695925.109. Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, et al. Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch Gen Psychiatry. 1999; 56:527–533.110. Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996; 58:1475–1483.111. Chetty S, Friedman AR, Taravosh-Lahn K, Kirby ED, Mirescu C, Guo F, et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry. 2014; 19:1275–1283.112. Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008; 29:367–374.113. Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010; 48:1121–1132.114. Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, et al. Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardiol. 2018; 71:263–275.115. El-Menyar AA. Cytokines and myocardial dysfunction: state of the art. J Card Fail. 2008; 14:61–74.116. Toledo C, Andrade DC, Díaz HS, Inestrosa NC, Del Rio R. Neurocognitive disorders in heart failure: novel pathophysiological mechanisms underpinning memory loss and learning impairment. Mol Neurobiol. 2019; 56:8035–8051.117. Hong X, Bu L, Wang Y, Xu J, Wu J, Huang Y, et al. Increases in the risk of cognitive impairment and alterations of cerebral β-amyloid metabolism in mouse model of heart failure. PLoS One. 2013; 8:e63829.118. Ferketich AK, Ferguson JP, Binkley PF. Depressive symptoms and inflammation among heart failure patients. Am Heart J. 2005; 150:132–136.119. Parissis JT, Adamopoulos S, Rigas A, Kostakis G, Karatzas D, Venetsanou K, et al. Comparison of circulating proinflammatory cytokines and soluble apoptosis mediators in patients with chronic heart failure with versus without symptoms of depression. Am J Cardiol. 2004; 94:1326–1328.120. Olmos G, Lladó J. Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014; 2014:861231.121. Gemma C, Bickford PC. Interleukin-1beta and caspase-1: players in the regulation of age-related cognitive dysfunction. Rev Neurosci. 2007; 18:137–148.122. Wright CB, Sacco RL, Rundek T, Delman J, Rabbani L, Elkind M. Interleukin-6 is associated with cognitive function: the Northern Manhattan study. J Stroke Cerebrovasc Dis. 2006; 15:34–38.123. Neri M, Fineschi V, Di Paolo M, Pomara C, Riezzo I, Turillazzi E, et al. Cardiac oxidative stress and inflammatory cytokines response after myocardial infarction. Curr Vasc Pharmacol. 2015; 13:26–36.124. Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012; 8:1254–1266.125. Skelly DT, Hennessy E, Dansereau MA, Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, [corrected] TNF-α and IL-6 challenges in C57BL/6 mice. PLoS One. 2013; 8:e69123.126. Bascuñana P, Hess A, Borchert T, Wang Y, Wollert KC, Bengel FM, et al. 11C-methionine PET identifies astroglia involvement in heart-brain inflammation networking after acute myocardial infarction. J Nucl Med. 2020; 61:977–980.127. Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015; 16:358–372.128. Smith JA, Das A, Ray SK, Banik NL. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res Bull. 2012; 87:10–20.129. Gruhl SL, Su J, Chua WC, Tay KV. Takotsubo cardiomyopathy in post-traumatic brain injury: a systematic review of diagnosis and management. Clin Neurol Neurosurg. 2022; 213:107119.130. Haghi D, Papavassiliu T, Heggemann F, Kaden JJ, Borggrefe M, Suselbeck T. Incidence and clinical significance of left ventricular thrombus in tako-tsubo cardiomyopathy assessed with echocardiography. QJM. 2008; 101:381–386.131. Ding KJ, Cammann VL, Szawan KA, Stähli BE, Wischnewsky M, Di Vece D, et al. Intraventricular thrombus formation and embolism in takotsubo syndrome: insights from the international takotsubo registry. Arterioscler Thromb Vasc Biol. 2020; 40:279–287.132. El-Battrawy I, Borggrefe M, Akin I. Takotsubo syndrome and embolic events. Heart Fail Clin. 2016; 12:543–550.133. Mitsuma W, Kodama M, Ito M, Kimura S, Tanaka K, Hoyano M, et al. Thromboembolism in takotsubo cardiomyopathy. Int J Cardiol. 2010; 139:98–100.134. Naegele M, Flammer AJ, Enseleit F, Roas S, Frank M, Hirt A, et al. Endothelial function and sympathetic nervous system activity in patients with takotsubo syndrome. Int J Cardiol. 2016; 224:226–230.135. Steffel J, Lüscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006; 113:722–731.136. Mehta JL, Calcaterra G, Bassareo PP. COVID-19, thromboembolic risk, and Virchow’s triad: lesson from the past. Clin Cardiol. 2020; 43:1362–1367.137. Pirzer R, Elmas E, Haghi D, Lippert C, Kralev S, Lang S, et al. Platelet and monocyte activity markers and mediators of inflammation in takotsubo cardiomyopathy. Heart Vessels. 2012; 27:186–192.138. Pyo MK, Yun-Choi HS, Hong YJ. Apparent heterogeneous responsiveness of human platelet rich plasma to catecholamines. Platelets. 2003; 14:171–178.139. Damås JK, Waehre T, Yndestad A, Otterdal K, Hognestad A, Solum NO, et al. Interleukin-7-mediated inflammation in unstable angina: possible role of chemokines and platelets. Circulation. 2003; 107:2670–2676.140. Schneider B, Athanasiadis A, Schwab J, Pistner W, Gottwald U, Schoeller R, et al. Complications in the clinical course of takotsubo cardiomyopathy. Int J Cardiol. 2014; 176:199–205.141. Mollet NR, Dymarkowski S, Volders W, Wathiong J, Herbots L, Rademakers FE, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation. 2002; 106:2873–2876.142. Abanador-Kamper N, Kamper L, Wolfertz J, Pomjanski W, Wolf-Pütz A, Seyfarth M. Evaluation of therapy management and outcome in takotsubo syndrome. BMC Cardiovasc Disord. 2017; 17:225.143. Rathore SS, Iqbal K, Shafqat S, Tariq E, Tousif S, UlHaq ZG, et al. Meta-analysis of incidence and outcomes of life-threatening arrhythmias in takotsubo cardiomyopathy. Indian Heart J. 2022; 74:110–119.144. El-Battrawy I, Lang S, Ansari U, Behnes M, Hillenbrand D, Schramm K, et al. Impact of concomitant atrial fibrillation on the prognosis of takotsubo cardiomyopathy. Europace. 2017; 19:1288–1292.145. Stiermaier T, Santoro F, Eitel C, Graf T, Möller C, Tarantino N, et al. Prevalence and prognostic relevance of atrial fibrillation in patients with takotsubo syndrome. Int J Cardiol. 2017; 245:156–161.146. Debonnaire P, Joyce E, Hiemstra Y, Mertens BJ, Atsma DE, Schalij MJ, et al. Left atrial size and function in hypertrophic cardiomyopathy patients and risk of new-onset atrial fibrillation. Circ Arrhythm Electrophysiol. 2017; 10:e004052.147. Fonseca AC, Almeida AG, Santos MO, Ferro JM. Neurological complications of cardiomyopathies. Handb Clin Neurol. 2021; 177:91–109.148. Dias A, Franco E, Janzer S, Koshkelashvili N, Bhalla V, Rubio M, et al. Incidence and predictors of stroke during the index event in an ethnically diverse takotsubo cardiomyopathy population. Funct Neurol. 2016; 31:157–162.149. Abe T, Olanipekun T, Igwe J, Khoury M, Busari O, MusongeEffoe J, et al. Trends, predictors and outcomes of ischemic stroke among patients hospitalized with takotsubo cardiomyopathy. J Stroke Cerebrovasc Dis. 2021; 30:106005.150. Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007; 38:981–986.151. Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D, et al. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012; 43:3230–3237.152. van der Jagt M. Fluid management of the neurological patient: a concise review. Crit Care. 2016; 20:126.153. Mutoh T, Kazumata K, Ishikawa T, Terasaka S. Performance of bedside transpulmonary thermodilution monitoring for goaldirected hemodynamic management after subarachnoid hemorrhage. Stroke. 2009; 40:2368–2374.154. Yoneda H, Nakamura T, Shirao S, Tanaka N, Ishihara H, Suehiro E, et al. Multicenter prospective cohort study on volume management after subarachnoid hemorrhage: hemodynamic changes according to severity of subarachnoid hemorrhage and cerebral vasospasm. Stroke. 2013; 44:2155–2161.155. Wang TD, Wu CC, Lee YT. Myocardial stunning after cerebral infarction. Int J Cardiol. 1997; 58:308–311.156. Sung PH, Chen KH, Lin HS, Chu CH, Chiang JY, Yip HK. The correlation between severity of neurological impairment and left ventricular function in patients after acute ischemic stroke. J Clin Med. 2019; 8:190.157. Bairashevskaia AV, Belogubova SY, Kondratiuk MR, Rudnova DS, Sologova SS, Tereshkina OI, et al. Update of takotsubo cardiomyopathy: present experience and outlook for the future. Int J Cardiol Heart Vasc. 2022; 39:100990.158. Abu Ghalyoun B, Guragai N, Garris R, Shamoon D, Chuang A, Shamoon F. Abstract 11626: faster recovery in takotsubo with prior beta-blocker use. Circulation. 2019; 140(Suppl_1):A11626.159. Isogai T, Matsui H, Tanaka H, Fushimi K, Yasunaga H. Early β-blocker use and in-hospital mortality in patients with takotsubo cardiomyopathy. Heart. 2016; 102:1029–1035.160. Brunetti ND, Santoro F, De Gennaro L, Correale M, Gaglione A, Di Biase M. Drug treatment rates with beta-blockers and ACE-inhibitors/angiotensin receptor blockers and recurrences in takotsubo cardiomyopathy: a meta-regression analysis. Int J Cardiol. 2016; 214:340–342.161. Laowattana S, Oppenheimer SM. Protective effects of betablockers in cerebrovascular disease. Neurology. 2007; 68:509–514.162. Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998; 98:1329–1334.163. Neil-Dwyer G, Walter P, Cruickshank JM, Doshi B, O’Gorman P. Effect of propranolol and phentolamine on myocardial necrosis after subarachnoid haemorrhage. Br Med J. 1978; 2:990–992.164. Alali AS, Mukherjee K, McCredie VA, Golan E, Shah PS, Bardes JM, et al. Beta-blockers and traumatic brain injury: a systematic review and meta-analysis. Ann Surg. 2017; 266:952–961.165. Ley EJ, Leonard SD, Barmparas G, Dhillon NK, Inaba K, Salim A, et al. Beta blockers in critically ill patients with traumatic brain injury: results from a multicenter, prospective, observational American Association for the Surgery of Trauma study. J Trauma Acute Care Surg. 2018; 84:234–244.166. Luo H, Song WX, Jiang JW, Zhao JL, Rong WL, Li MH. Effects of preadmission beta-blockers on neurogenic stunned myocardium after aneurysmal subarachnoid hemorrhage: a metaanalysis. Clin Neurol Neurosurg. 2017; 158:77–81.167. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med. 2004; 141:858–865.168. Calviello LA, Donnelly J, Zeiler FA, Thelin EP, Smielewski P, Czosnyka M. Cerebral autoregulation monitoring in acute traumatic brain injury: what’s the evidence? Minerva Anestesiol. 2017; 83:844–857.169. Talahma M, Alkhachroum AM, Alyahya M, Manjila S, Xiong W. Takotsubo cardiomyopathy in aneurysmal subarachnoid hemorrhage: institutional experience and literature review. Clin Neurol Neurosurg. 2016; 141:65–70.170. Wagner S, Güthe T, Bhogal P, Cimpoca A, Ganslandt O, Bäzner H, et al. Aneurysmal subarachnoid hemorrhage as a trigger for takotsubo syndrome: a comprehensive review. Rev Cardiovasc Med. 2021; 22:1241–1251.171. Omerovic E. Takotsubo syndrome-scientific basis for current treatment strategies. Heart Fail Clin. 2016; 12:577–586.172. Nalluri N, Asti D, Anugu VR, Ibrahim U, Lafferty JC, Olkovsky Y. Cardiogenic shock secondary to takotsubo cardiomyopathy in a patient with preexisting hypertrophic obstructive cardiomyopathy. CASE (Phila). 2018; 2:78–81.173. Jha S, Zeijlon R, Shekka Espinosa A, Alkhoury J, Oras J, Omerovic E, et al. Clinical management in the takotsubo syndrome. Expert Rev Cardiovasc Ther. 2019; 17:83–93.174. du Toit EF, Genis A, Opie LH, Pollesello P, Lochner A. A role for the RISK pathway and K(ATP) channels in pre- and post-conditioning induced by levosimendan in the isolated guinea pig heart. Br J Pharmacol. 2008; 154:41–50.175. Taccone FS, Brasseur A, Vincent JL, De Backer D. Levosimendan for the treatment of subarachnoid hemorrhage-related cardiogenic shock. Intensive Care Med. 2013; 39:1497–1498.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stress-Induced Cardiomyopathy: The Role of Echocardiography
- Heart Failure as a Risk Factor for Stroke
- Stress Cardiomyopathy due to Misuse of Transdermal Fentanyl Patches in an Elderly Patient
- A Case of Stress-Induced Cardiomyopathy in Sheehan's Syndrome
- Stress-induced cardiomyopathy during ophthalmologic surgery: A case report