Kosin Med J.
2022 Sep;37(3):242-248. 10.7180/kmj.22.116.
The clinical significance of circulating microRNA-21 in patients with IgA nephropathy
- Affiliations
-
- 1Division of Nephrology, Department of Internal Medicine, Presbyterian Medical Center, Jeonju, Korea
- 2Christian Medical Research Center, Presbyterian Medical Center, Jeonju, Korea
- KMID: 2538804
- DOI: http://doi.org/10.7180/kmj.22.116
Abstract
- Background
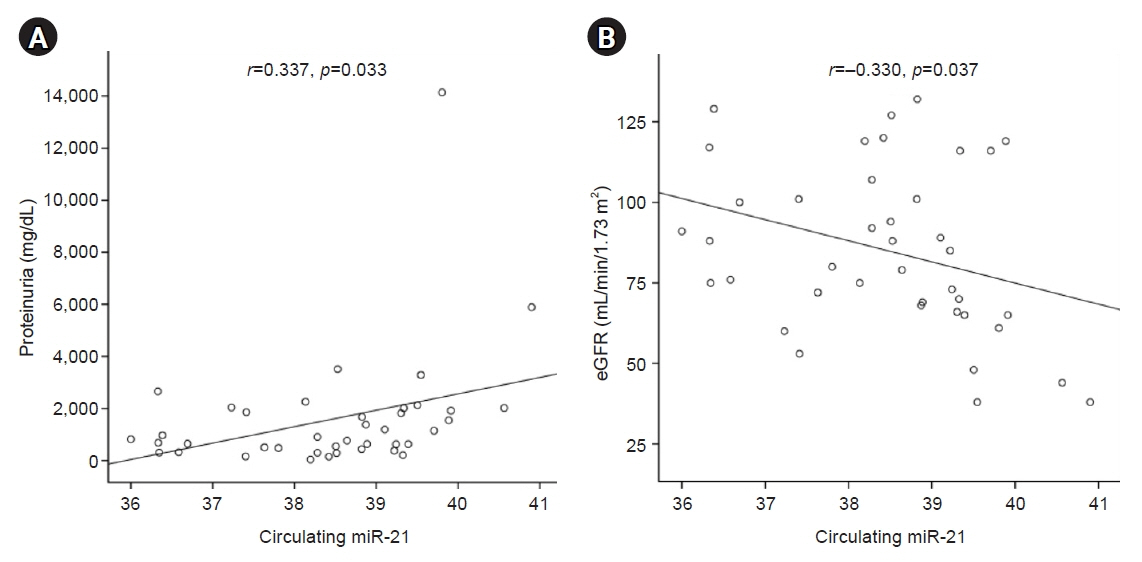
Urinary microRNA-21 (miR-21) has been reported to correlate with the histologic lesions of IgA nephropathy (IgAN). We investigated whether urinary or circulating miR-21 could serve as a biomarker for detecting the renal progression of IgAN.
Methods
Forty patients with biopsy-proven IgAN were enrolled in this study. Serum and urinary sediment miRs were extracted, and the expression of miR-21 was quantified by real-time quantitative polymerase chain reaction. Renal progression was defined as end-stage renal disease, a sustained doubling of serum creatinine, or a 50% decrease in estimated glomerular filtration rate (eGFR) from baseline.
Results
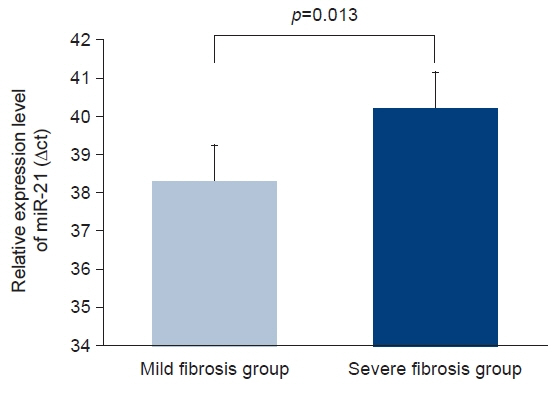
Six patients experienced renal progression during the follow-up period. The baseline eGFR was lower in the progression group (49±11 mL/min/1.73 m2 vs. 90±23 mL/min/1.73 m2, p<0.05) than in the non-progression group. The level of circulating miR-21 on kidney biopsy was higher in the progression group than in the non-progression group (40.0±0.6 vs. 38.2±1.1 ΔCt value of miR-21, p<0.01), whereas there was no significant difference in urinary miR-21 (38.1±2.1 vs. 37.8±1.4 ΔCt value of miR-21, p=0.687) between the two groups. Receiver operating characteristic curve analysis demonstrated that circulating miR-21 had good discriminative power for diagnosing renal progression of IgAN, with an area under the curve of 0.975.
Conclusions
The level of circulating miR-21 was higher in the progression group than in the non-progression group at the time of kidney biopsy. Therefore, circulating miR-21 could be a surrogate marker of renal progression in patients with IgAN.
Keyword
Figure
Reference
-
References
1. Moriyama T, Tanaka K, Iwasaki C, Oshima Y, Ochi A, Kataoka H, et al. Prognosis in IgA nephropathy: 30-year analysis of 1,012 patients at a single center in Japan. PLoS One. 2014; 9:e91756.2. Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant. 2012; 27:1479–85.3. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76:546–56.4. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008; 9:102–14.5. Sun IO, Lerman LO. Urinary microRNA in kidney disease: utility and roles. Am J Physiol Renal Physiol. 2019; 316:F785–93.6. Xu BY, Meng SJ, Shi SF, Liu LJ, Lv JC, Zhu L, et al. MicroRNA-21-5p participates in IgA nephropathy by driving T helper cell polarization. J Nephrol. 2020; 33:551–60.7. Liang S, Cai GY, Duan ZY, Liu SW, Wu J, Lv Y, et al. Urinary sediment miRNAs reflect tubulointerstitial damage and therapeutic response in IgA nephropathy. BMC Nephrol. 2017; 18:63.8. Szeto CC, Wang G, Ng JK, Kwan BC, Mac-Moune Lai F, Chow KM, et al. Urinary miRNA profile for the diagnosis of IgA nephropathy. BMC Nephrol. 2019; 20:77.9. Chung AC, Dong Y, Yang W, Zhong X, Li R, Lan HY. Smad7 suppresses renal fibrosis via altering expression of TGF-β/Smad3-regulated microRNAs. Mol Ther. 2013; 21:388–98.10. Lan HY, Chung AC. TGF-β/Smad signaling in kidney disease. Semin Nephrol. 2012; 32:236–43.11. Trionfini P, Benigni A, Remuzzi G. MicroRNAs in kidney physiology and disease. Nat Rev Nephrol. 2015; 11:23–33.12. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007; 53:766–72.13. Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017; 91:1014–21.14. Park MY, Herrmann SM, Saad A, Widmer RJ, Tang H, Zhu XY, et al. Circulating and renal vein levels of microRNAs in patients with renal artery stenosis. Nephrol Dial Transplant. 2015; 30:480–90.15. Xu X, Kriegel AJ, Jiao X, Liu H, Bai X, Olson J, et al. miR-21 in ischemia/reperfusion injury: a double-edged sword? Physiol Genomics. 2014; 46:789–97.16. Sole C, Moline T, Vidal M, Ordi-Ros J, Cortes-Hernandez J. An exosomal urinary miRNA signature for early diagnosis of renal fibrosis in lupus nephritis. Cells. 2019; 8:773.17. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008; 105:10513–8.18. Lopes CB, Magalhaes LL, Teofilo CR, Alves APNN, Montenegro RC, Negrini M, et al. Differential expression of hsa-miR-221, hsa-miR-21, hsa-miR-135b, and hsa-miR-29c suggests a field effect in oral cancer. BMC Cancer. 2018; 18:721.19. Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci U S A. 2011; 108:10355–60.20. Fouad M, Salem I, Elhefnawy K, Raafat N, Faisal A. MicroRNA-21 as an early marker of nephropathy in patients with type 1 diabetes. Indian J Nephrol. 2020; 30:21–5.21. Yun CY, Lim JH, Oh JH, Cho AY, Lee KY, Sun IO. Urinary exosomal microRNA-21 as a marker for scrub typhus-associated acute kidney injury. Genet Test Mol Biomarkers. 2021; 25:140–4.22. Wang N, Zhou Y, Jiang L, Li D, Yang J, Zhang CY, et al. Urinary microRNA-10a and microRNA-30d serve as novel, sensitive and specific biomarkers for kidney injury. PLoS One. 2012; 7:e51140.23. Wang Z, Liao Y, Wang L, Lin Y, Ye Z, Zeng X, et al. Small RNA deep sequencing reveals novel miRNAs in peripheral blood mononuclear cells from patients with IgA nephropathy. Mol Med Rep. 2020; 22:3378–86.24. Ramezani A, Devaney JM, Cohen S, Wing MR, Scott R, Knoblach S, et al. Circulating and urinary microRNA profile in focal segmental glomerulosclerosis: a pilot study. Eur J Clin Invest. 2015; 45:394–404.25. Sun IO, Bae YU, Lee H, Kim H, Jeon JS, Noh H, et al. Circulating miRNAs in extracellular vesicles related to treatment response in patients with idiopathic membranous nephropathy. J Transl Med. 2022; 20:224.26. Hu H, Wan Q, Li T, Qi D, Dong X, Xu Y, et al. Circulating MiR-29a, possible use as a biomarker for monitoring IgA nephropathy. Iran J Kidney Dis. 2020; 14:107–18.27. Li H, Chen Z, Chen W, Li J, Liu Y, Ma H, et al. MicroRNA-23b-3p deletion induces an IgA nephropathy-like disease associated with dysregulated mucosal IgA synthesis. J Am Soc Nephrol. 2021; 32:2561–78.28. Tan K, Chen J, Li W, Chen Y, Sui W, Zhang Y, et al. Genome-wide analysis of microRNAs expression profiling in patients with primary IgA nephropathy. Genome. 2013; 56:161–9.29. Pawluczyk IZA, Didangelos A, Barbour SJ, Er L, Becker JU, Martin R, et al. Differential expression of microRNA miR-150-5p in IgA nephropathy as a potential mediator and marker of disease progression. Kidney Int. 2021; 99:1127–39.30. Serino G, Sallustio F, Cox SN, Pesce F, Schena FP. Abnormal miR-148b expression promotes aberrant glycosylation of IgA1 in IgA nephropathy. J Am Soc Nephrol. 2012; 23:814–24.31. Serino G, Pesce F, Sallustio F, De Palma G, Cox SN, Curci C, et al. In a retrospective international study, circulating miR-148b and let-7b were found to be serum markers for detecting primary IgA nephropathy. Kidney Int. 2016; 89:683–92.32. Ono S, Lam S, Nagahara M, Hoon DS. Circulating microRNA biomarkers as liquid biopsy for cancer patients: pros and cons of current assays. J Clin Med. 2015; 4:1890–907.33. Roberts TC, Coenen-Stass AM, Wood MJ. Assessment of RT-qPCR normalization strategies for accurate quantification of extracellular microRNAs in murine serum. PLoS One. 2014; 9:e89237.