J Yeungnam Med Sci.
2023 Jan;40(1):65-77. 10.12701/jyms.2022.00213.
The effect and therapeutic compliance of adjuvant therapy in patients with cholangiocarcinoma after R0 resection: a retrospective study
- Affiliations
-
- 1Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- KMID: 2538787
- DOI: http://doi.org/10.12701/jyms.2022.00213
Abstract
- Background
This study aimed to compare clinical outcomes between surveillance and adjuvant therapy (AT) groups after R0 resection for cholangiocarcinoma (CCA).
Methods
A total of 154 patients who underwent R0 resection for CCA at the Daegu Catholic University Medical Center between January 2010 and December 2019 were included. Overall survival (OS) and progression-free survival (PFS) were analyzed.
Results
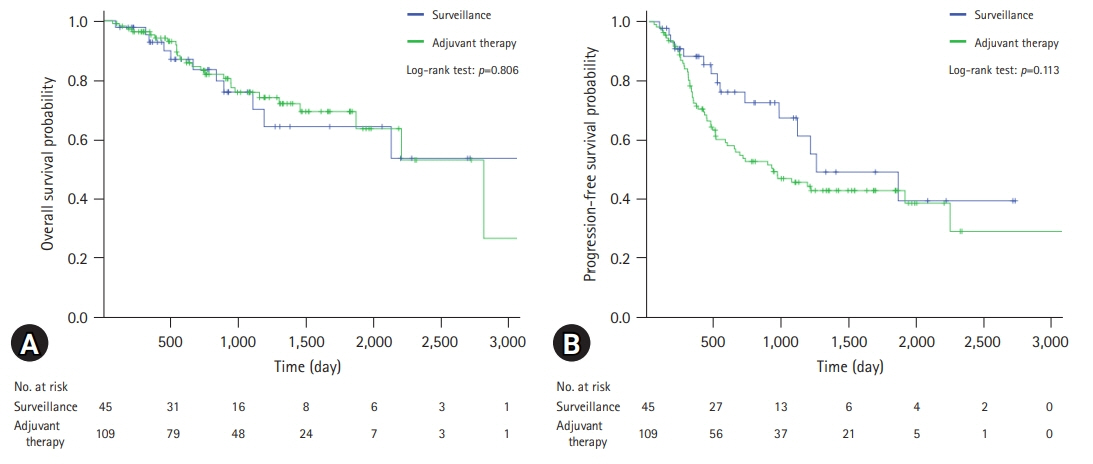
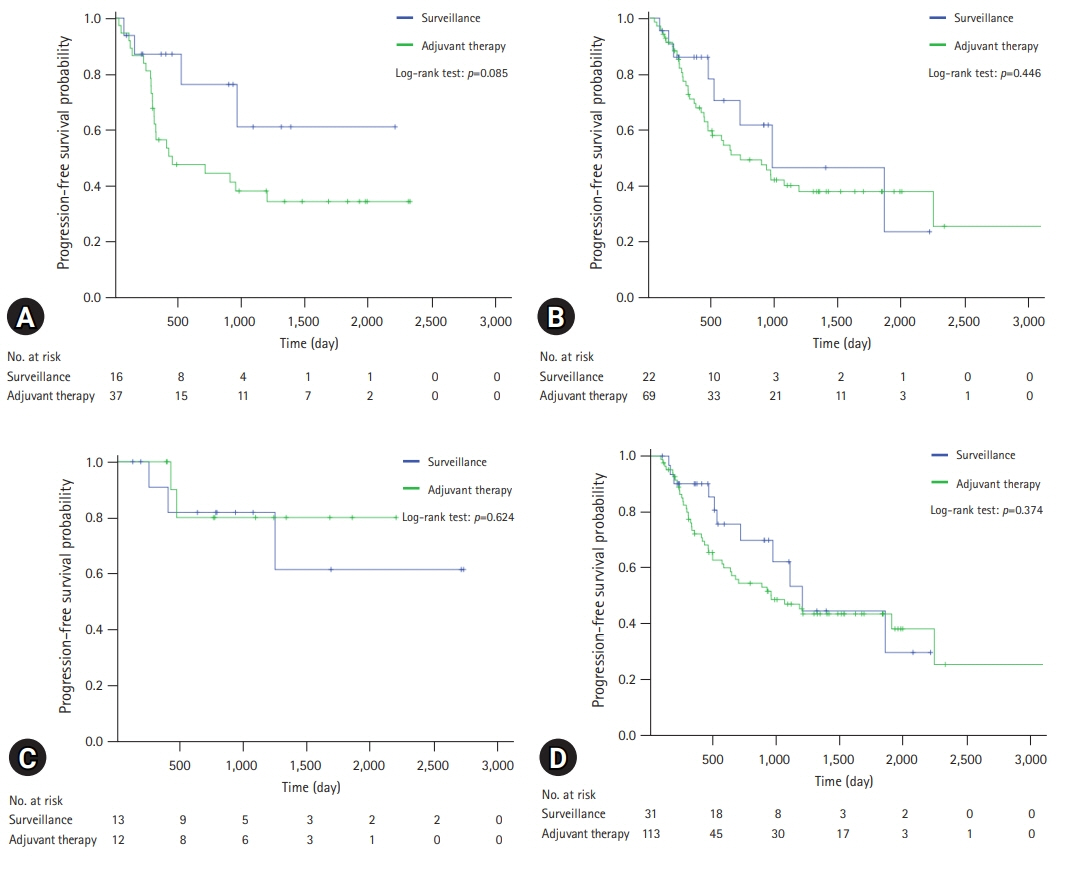
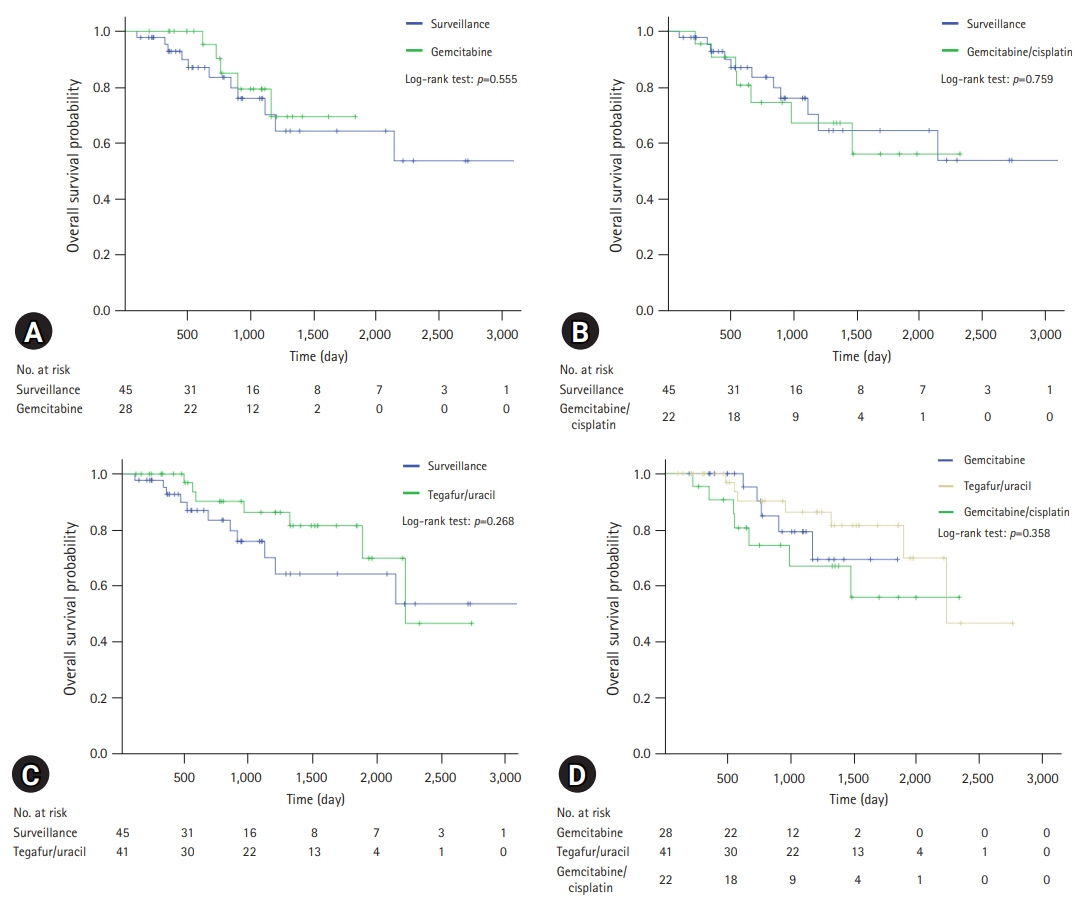
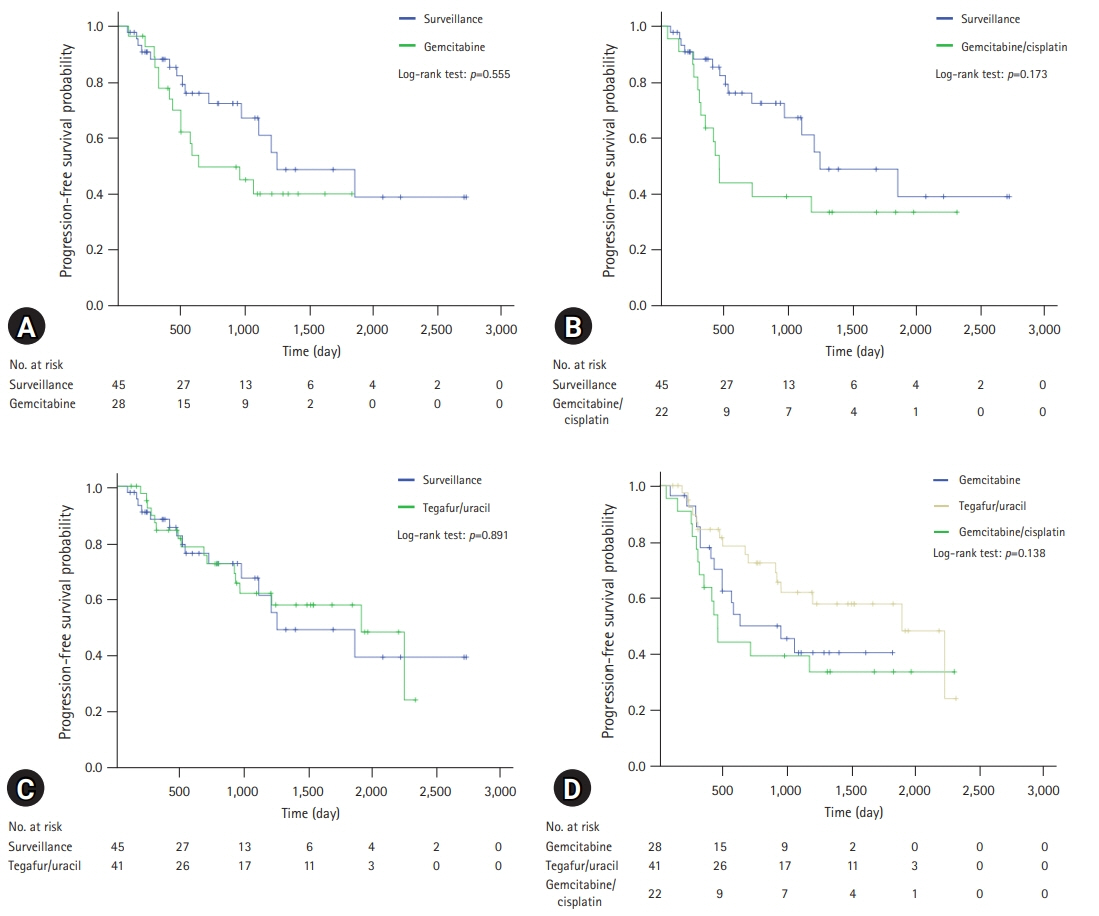
The median follow-up duration was 899 days. There were 109 patients in the AT group and 45 patients in the surveillance group. The patients in the AT group were younger (67 years vs. 74 years, p<0.001) and included more males (64.2% vs. 46.7%, p=0.044). The proportion of patients with stage III CCA was larger in the AT group than in the surveillance group (13.8% vs. 2.2%, p=0.005). In addition, AT did not improve OS (5-year OS rate, 69.3% in the AT group vs. 64.2% in the surveillance group, p=0.806) or PFS (5-year PFS rate, 42.6% in the AT group vs. 48.9% in the surveillance group, p=0.113). In multivariate analysis using the Cox proportional hazards model, stage III CCA (hazard ratio [HR], 10.81; 95% confidence interval [CI], 2.92–40.00; p<0.001) was a significant predictor of OS. American Society of Anesthesiologists classification II (HR, 0.50; 95% CI, 0.31–0.81; p=0.005), and American Joint Committee on Cancer stages II (HR, 3.14; 95% CI, 1.25–7.89; p=0.015) and III (HR, 8.08; 95% CI, 2.80–23.32; p<0.001) were independent predictors of PFS.
Conclusion
AT after R0 resection for CCA did not improve OS or PFS.
Keyword
Figure
Reference
-
References
1. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primers. 2021; 7:65.2. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019; 39(Suppl 1):19–31.3. Belkouz A, Wilmink JW, Haj Mohammad N, Hagendoorn J, de Vos-Geelen J, Dejong CH, et al. Advances in adjuvant therapy of biliary tract cancer: an overview of current clinical evidence based on phase II and III trials. Crit Rev Oncol Hematol. 2020; 151:102975.4. Allen MJ, Knox JJ. A review of current adjuvant and neoadjuvant systemic treatments for cholangiocarcinoma and gallbladder carcinoma. Hepatoma Res. 2021; 7:73.5. Kim BW, Oh CM, Choi HY, Park JW, Cho H, Ki M. Incidence and overall survival of biliary tract cancers in South Korea from 2006 to 2015: using the National Health Information Database. Gut Liver. 2019; 13:104–13.6. Takada T, Amano H, Yasuda H, Nimura Y, Matsushiro T, Kato H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma?: a phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002; 95:1685–95.7. Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012; 308:147–56.8. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019; 20:663–73.9. Ebata T, Hirano S, Konishi M, Uesaka K, Tsuchiya Y, Ohtsuka M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018; 105:192–202.10. Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly JP, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol. 2019; 37:658–67.11. Kim YS, Jeong CY, Song HN, Kim TH, Kim HJ, Lee YJ, et al. The efficacy of fluoropyrimidine-based adjuvant chemotherapy on biliary tract cancer after R0 resection. Chin J Cancer. 2017; 36:9.12. McNamara MG, Walter T, Horgan AM, Amir E, Cleary S, McKeever EL, et al. Outcome of adjuvant therapy in biliary tract cancers. Am J Clin Oncol. 2015; 38:382–7.13. Miyata Y, Kogure R, Nakazawa A, Nagata R, Mitsui T, Ninomiya R, et al. The efficacy of S-1 as adjuvant chemotherapy for resected biliary tract carcinoma: a propensity score-matching analysis. J Clin Med. 2021; 10:925.14. Wang L, Deng M, Ke Q, Lou J, Zheng S, Bi X, et al. Postoperative adjuvant therapy following radical resection for intrahepatic cholangiocarcinoma: a multicenter retrospective study. Cancer Med. 2020; 9:2674–85.15. Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakamura H, et al. Adjuvant gemcitabine plus S-1 chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg. 2009; 250:950–6.16. Yamanaka K, Hatano E, Kanai M, Tanaka S, Yamamoto K, Narita M, et al. A single-center analysis of the survival benefits of adjuvant gemcitabine chemotherapy for biliary tract cancer. Int J Clin Oncol. 2014; 19:485–9.17. Kim YS, Hwang IG, Park SE, Go SI, Kang JH, Park I, et al. Role of adjuvant therapy after R0 resection for patients with distal cholangiocarcinoma. Cancer Chemother Pharmacol. 2016; 77:979–85.18. Kim YS, Oh SY, Go SI, Kang JH, Park I, Song HN, et al. The role of adjuvant therapy after R0 resection for patients with intrahepatic and perihilar cholangiocarcinomas. Cancer Chemother Pharmacol. 2017; 79:99–106.19. Bergeat D, Turrini O, Courtin-Tanguy L, Truant S, Darnis B, Delpero JR, et al. Impact of adjuvant chemotherapy after pancreaticoduodenectomy for distal cholangiocarcinoma: a propensity score analysis from a French multicentric cohort. Langenbecks Arch Surg. 2018; 403:701–9.20. Yin L, Xu Q, Li J, Wei Q, Ying J. The efficiency and regimen choice of adjuvant chemotherapy in biliary tract cancer: a STROBE-compliant retrospective cohort study. Medicine (Baltimore). 2018; 97:e13570.21. National Comprehensive Cancer Network (NCCN). NCCN Practice Guidelines in Oncology. Hepatobiliary cancers, version 5 [Internet]. Plymouth Meeting (PA): NCCN;2021. [cited 2021 Sep 21]. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf.22. Ringborg U. Adjuvant chemotherapy: a discussion of some basic principles. Acta Oncol. 1991; 30:251–3.23. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987; 317:1098.24. Saam J, Critchfield GC, Hamilton SA, Roa BB, Wenstrup RJ, Kaldate RR. Body surface area-based dosing of 5-fluoruracil results in extensive interindividual variability in 5-fluorouracil exposure in colorectal cancer patients on FOLFOX regimens. Clin Colorectal Cancer. 2011; 10:203–6.25. Capitain O, Asevoaia A, Boisdron-Celle M, Poirier AL, Morel A, Gamelin E. Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study. Clin Colorectal Cancer. 2012; 11:263–7.26. Chauhan A, House MG, Pitt HA, Nakeeb A, Howard TJ, Zyromski NJ, et al. Post-operative morbidity results in decreased long-term survival after resection for hilar cholangiocarcinoma. HPB (Oxford). 2011; 13:139–47.27. Hurwitz EE, Simon M, Vinta SR, Zehm CF, Shabot SM, Minhajuddin A, et al. Adding examples to the ASA-Physical Status Classification improves correct assignment to patients. Anesthesiology. 2017; 126:614–22.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant and Adjuvant Chemotherapy for Cholangiocarcinoma
- Analysis of Survival and Factors Affecting the Survival after Surgical Resection of Peripheral Cholangiocarcinoma: 318 Cases in Single Institute
- Preoperative Chemotherapy in Advanced Stomach Cancer (Pros)
- Current Updates in the Surgical Management of Hilar Cholangiocarcinoma
- Impact of Resection Margin Distance on Survival of Pancreatic Cancer: A Systematic Review and Meta-Analysis