Lab Med Online.
2022 Jul;12(3):169-174. 10.47429/lmo.2022.12.3.169.
Performance Evaluation of the AllCheck hCG Card Point-of-Care Device and Method Comparison with the Alere hCG Cassette Assay for Pregnancy Test
- Affiliations
-
- 1Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea
- 3Research Institute, Seoul Medical Center, Seoul, Korea
- KMID: 2538601
- DOI: http://doi.org/10.47429/lmo.2022.12.3.169
Abstract
- Background
Immunochromatographic point-of-care (POC) devices ae widely used by laboratories and lay users for urinary human chorionic gonadotropin (hCG) detection. Performance evaluation of pregnancy POC devices is rarely published. We performed an analytical and clinical validation of the newly introduced AllCheck hCG Card assay and compared it with the Alere hCG Cassette comparative assay.
Methods
The analytical performance of the assay was evaluated using an international standard material for hCG, as per the protocol recommended in the Clinical and Laboratory Standards Institute (CLSI) guideline. Clinical validation and comparison study with the comparative method were performed with remnant urine samples from pregnant and non-pregnant women.
Results
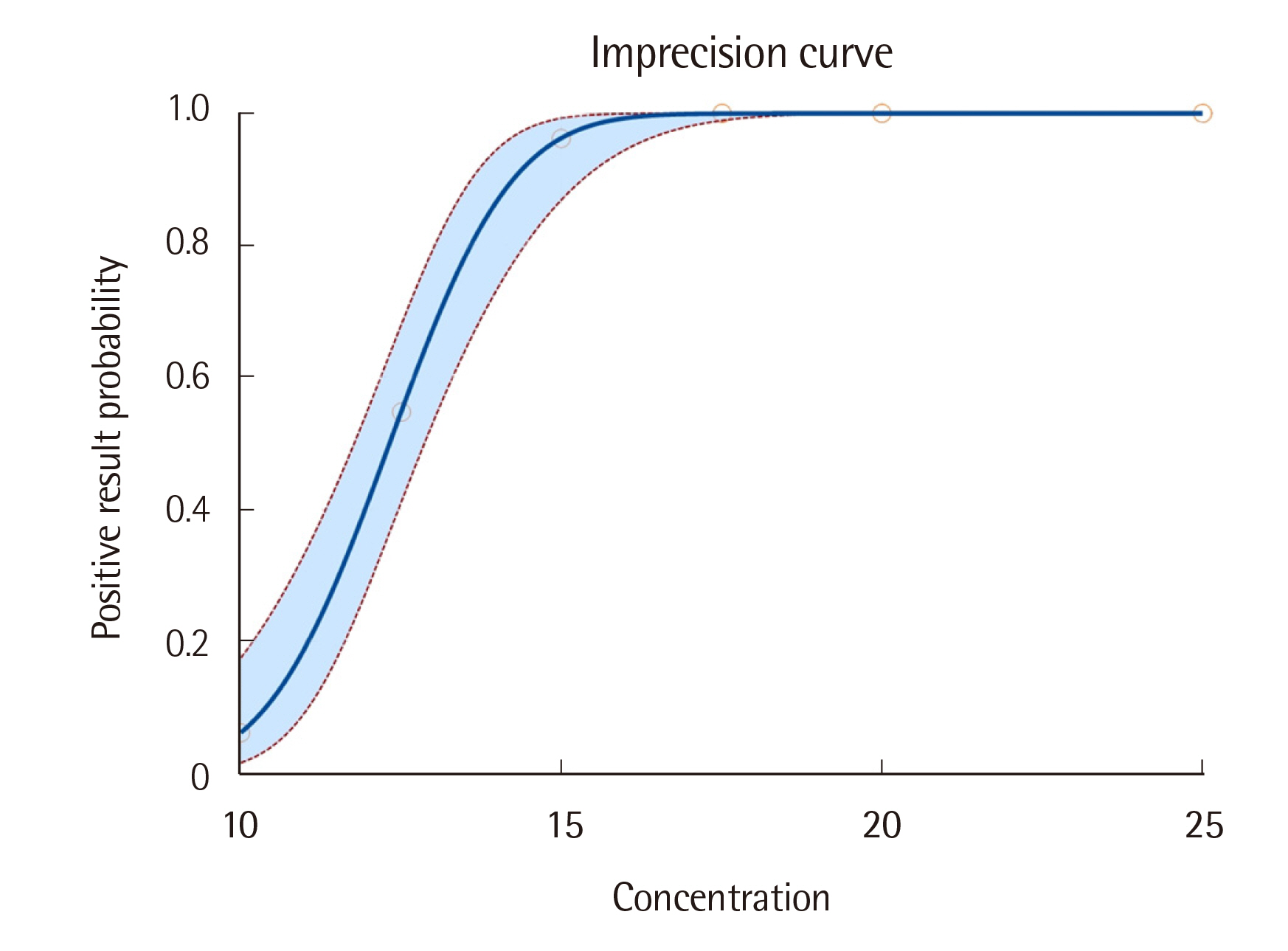
Probit analysis showed an analytical sensitivity of 15.82 mIU/mL. The precision of the assay was validated at a threshold of 30%. Cross-reactivity with luteinizing hormone, follicle-stimulating hormone, and thyroid-stimulating hormone was not observed. Comparison with the comparative assay showed a negative percent agreement of 100.0% (95% confidence interval [CI]: 92.9%-100.0%) and a positive percent agreement of 96.4% (95% CI: 89.9%-98.8%). Cohen’s kappa value was 0.952 (95% CI: 0.899-1.000).
Conclusions
Overall, we validated the performance of the urine hCG POC device and suggest that probit regression is suitable for qualitative tests other than molecular tests. The AllCheck hCG Card device satisfied the demanding standards suggested by the CLSI guideline and was suitable for clinical use.
Keyword
Figure
Reference
-
1. Stenman UH, Tiitinen A, Alfthan H, Valmu L. 2006; The classification, functions and clinical use of different isoforms of HCG. Hum Reprod Update. 12:769–84. DOI: 10.1093/humupd/dml029. PMID: 16877746.
Article2. Birken S, Armstrong EG, Kolks MA, Cole LA, Agosto GM, Krichevsky A, et al. 1988; Structure of the human chorionic gonadotropin beta-subunit fragment from pregnancy urine. Endocrinology. 123:572–83. DOI: 10.1210/endo-123-1-572. PMID: 2454811.
Article3. Kovalevskaya G, Kakuma T, Schlatterer J, O'Connor JF. 2007; Hyperglycosylated HCG expression in pregnancy: cellular origin and clinical applications. Mol Cell Endocrinol. 260-262:237–43. DOI: 10.1016/j.mce.2006.02.021. PMID: 17092638.
Article4. Norman RJ, Menabawey M, Lowings C, Buck RH, Chard T. 1987; Relationship between blood and urine concentrations of intact human chorionic gonadotropin and its free subunits in early pregnancy. Obstet Gynecol. 69:590–3.5. Kato Y, Braunstein GD. 1988; Beta-core fragment is a major form of immunoreactive urinary chorionic gonadotropin in human pregnancy. J Clin Endocrinol Metab. 66:1197–201. DOI: 10.1210/jcem-66-6-1197. PMID: 2453528.6. Herskovits AZ, Chen Y, Latifi N, Ta RM, Kriegel G. 2020; False-negative urine human chorionic gonadotropin testing in the clinical laboratory. Lab Med. 51:86–93. DOI: 10.1093/labmed/lmz039. PMID: 31245816.
Article7. Cervinski MA, Lockwood CM, Ferguson AM, Odem RR, Stenman UH, Alfthan H, et al. 2009; Qualitative point-of-care and over-the-counter urine hCG devices differentially detect the hCG variants of early pregnancy. Clin Chim Acta. 406:81–5. DOI: 10.1016/j.cca.2009.05.018. PMID: 19477170.
Article8. Griffey RT, Trent CJ, Bavolek RA, Keeperman JB, Sampson C, Poirier RF. 2013; "Hook-like effect" causes false-negative point-of-care urine pregnancy testing in emergency patients. J Emerg Med. 44:155–60. DOI: 10.1016/j.jemermed.2011.05.032. PMID: 21835572.
Article9. Sturgeon C, Butler SA, Gould F, Johnson S, Rowlands S, Stenman UH, et al. 2021; Recommendations for validation testing of home pregnancy tests (HPTs) in Europe. Clin Chem Lab Med. 59:823–35. DOI: 10.1515/cclm-2020-1523. PMID: 33544509.
Article10. Clinical, Laboratory Standards Institute. 2008. User protocol for evaluation of qualitative test performance; approved guideline-second edition. CLSI document EP12-A2. Clinical and Laboratory Standards Institute;Wayne, PA:11. Kamer SM, Foley KF, Schmidt RL, Greene DN. 2015; Analytical sensitivity of four commonly used hCG point of care devices. Clin Biochem. 48:448–52. DOI: 10.1016/j.clinbiochem.2014.12.015. PMID: 25549977.
Article12. Clinical, Laboratory Standards Institute. 2012. Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline-second edition. CLSI document EP17-A2. Clinical and Laboratory Standards Institute;Wayne, PA:
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Four Point-of-Care Devices for Pregnancy Test in Urine
- Effect of beta-hCG with a Point-of-Care Test in the Emergency Department
- Evaluation of Enzyme Immunoassay for Urinary Pregnancy Tests
- Predictive Value of Serum beta-hCG Level in Pregnancies following In Vitro Fertilization and Embryo Transfer
- Diagnostic Value of Serum Beta-hCG Measured by EIA in Suspected Ectopic Pregnancy