Lab Med Online.
2022 Apr;12(2):129-133. 10.47429/lmo.2022.12.2.129.
A Case of Monoclonal Gammopathy Associated with Aplastic Anemia: Insights into the Etiology and Points to be Considered During Diagnosis
- Affiliations
-
- 1Department of Laboratory Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
- 2Department of Laboratory Medicine, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea
- KMID: 2538596
- DOI: http://doi.org/10.47429/lmo.2022.12.2.129
Abstract
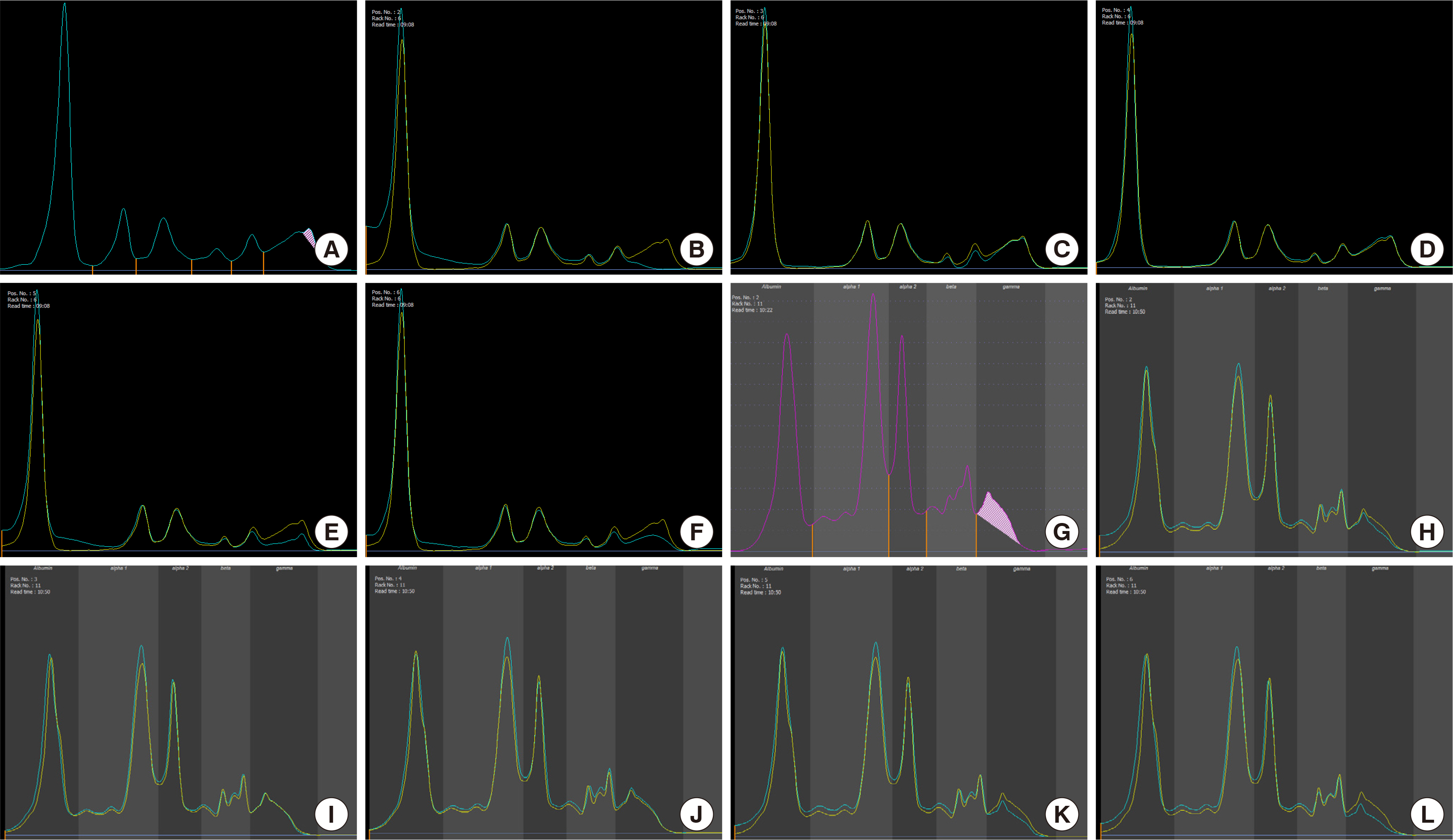
- Monoclonal gammopathy involves the presence of clonal immunoglobulins, fragments, or free light chains of immunoglobulin in the serum or urine, and is usually considered a sign of plasma cell or B cell malignancies. However, monoclonal gammopathy could also be benign. Here, we present a case of monoclonal gammopathy associated with aplastic anemia, the diagnostic steps, and possible etiologies. Evaluating the cause of monoclonal gammopathy in hypocellular bone marrow could be challenging due to the high proportion of plasma cell and rarity of the combination of these two conditions. Therefore, extensive diagnostic analysis and follow-up tests are necessary to conduct accurate diagnosis.
Keyword
Figure
Reference
-
1. Alexanian R, Weber D, Liu F. 1999; Differential diagnosis of monoclonal Gammopathies. Arch Pathol Lab Med. 123:108–13. DOI: 10.5858/1999-123-0108-DDOMG. PMID: 10050782.
Article2. Gerritsen E, Vossen J, Van Tol M, Jol-Van Der Zijde C, Van Der Weijden-Ragas R, Radl J. 1989; Monoclonal gammopathies in children. J Clin Immunol. 9:296–305. DOI: 10.1007/BF00918661. PMID: 2504763.
Article3. Strobel SL. 2003; Transient paraproteinemia: an intriguing immunological anomaly. Ann Clin Lab Sci. 33:265–70. PMID: 12956440.4. Vazzana N, Ognibene S, Dipaola F. 2020; "Acute" monoclonal gammopathy in severe COVID-19. Hematol Transfus Cell Ther. 42:218–20. DOI: 10.1016/j.htct.2020.05.002. PMID: 32546369. PMCID: PMC7280116.
Article5. Widmer CC, Balabanov S, Schanz U, Theocharides APA. 2017; Transient paraproteinemia after allogeneic hematopoietic stem cell transplantation is an underexplored phenomenon associated with graft versus host disease. Oncotarget. 8:106333–41. DOI: 10.18632/oncotarget.22462. PMID: 29290952. PMCID: PMC5739737.
Article6. Yokoh K, Kimura S, Wada K, Morita Y, Ozawa M, Kobayashi Y, et al. 1990; Aplastic anemia associated with minimal serum M-protein treated successfully with repeated bolus methylprednisolone therapy. Rinsho Ketsueki. 31:506–10. PMID: 2381070.7. Shallis RM, Ahmad R, Zeidan AM. 2018; Aplastic anemia: Etiology, molecular pathogenesis, and emerging concepts. Eur J Haematol. 101:711–20. DOI: 10.1111/ejh.13153. PMID: 30055055.
Article8. Swerdlow SH, Campo E, editors. 2017. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon, France: IARC;p. 242.