J Korean Med Sci.
2023 Jan;38(3):e16. 10.3346/jkms.2023.38.e16.
Radiologic Subtypes and Treatment Outcome of Unclassifiable Type Mycobacterium avium Complex Pulmonary Disease
- Affiliations
-
- 1Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Infectious Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2538371
- DOI: http://doi.org/10.3346/jkms.2023.38.e16
Abstract
- Background: Although the “unclassifiable type” is categorized as one of the radiologic classifications in Mycobacterium avium complex (MAC) pulmonary disease (PD), there have been few studies of this type thus far. We aimed to investigate the radiologic subtypes and treatment outcome of unclassifiable type MAC-PD.
Methods
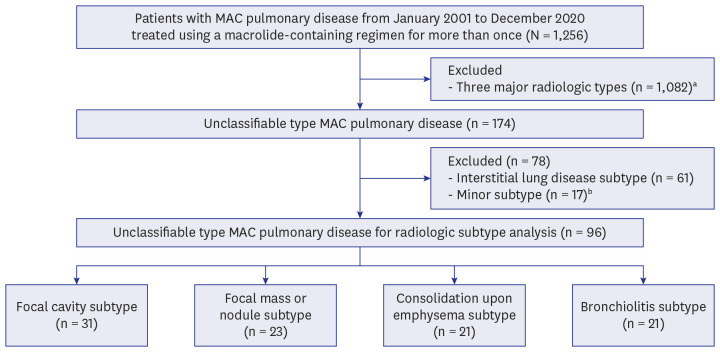
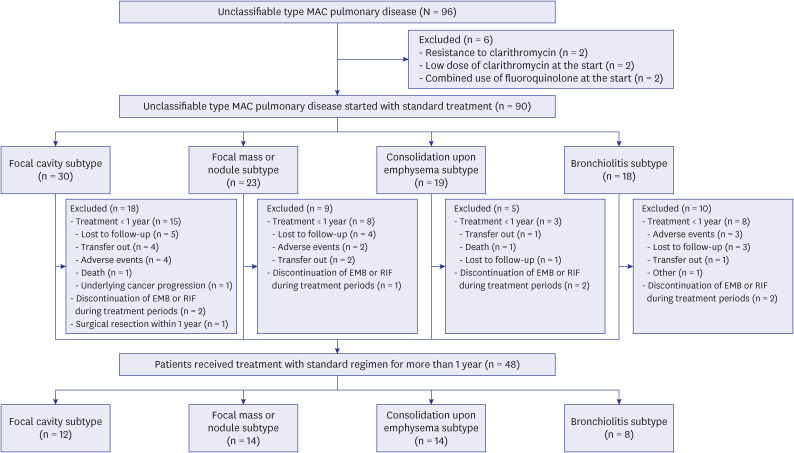
Ninety-six patients with unclassifiable type MAC-PD who initiated a macrolidecontaining regimen from 2001 to 2020 were identified at a tertiary referral center in South Korea. Among these 96 patients, 1-year culture conversion rate was analyzed for 48 patients who received standard treatment (three-drug oral-antibiotic combination with or without an injectable agent) for ≥ 1 year.
Results
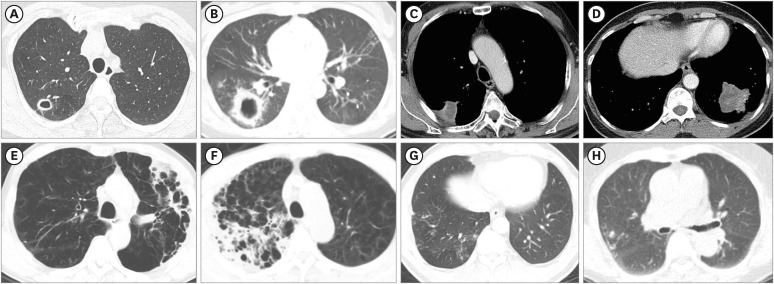
The mean age of the 96 patients was 65.4 ± 10.8 years, and 72.9% of them were male. These patients were classified into four major radiologic subtypes; the most common subtype was the focal cavity subtype (n = 31, 32.3%), followed by the focal mass or nodule (n = 23, 24.0%), consolidation upon emphysema (n = 21, 21.9%), and bronchiolitis (n = 21, 21.9%) subtypes. For the 48 patients who received standard treatment for ≥ 1 year, the overall rate of culture conversion at 1-year was 93.8%. All patients in the focal cavity subtype and focal mass or nodule subtype categories achieved 1-year culture conversion. Additionally, 1-year culture conversion rate was 92.9% in consolidation upon emphysema subtype and 75.0% in bronchiolitis subtype.
Conclusion
Unclassifiable type MAC-PD can be radiologically further categorized into four major radiologic subtypes. The treatment outcome of all of these subtypes seems to be favorable.
Figure
Reference
-
1. Kwon YS, Koh WJ, Daley CL. Treatment of Mycobacterium avium complex pulmonary disease. Tuberc Respir Dis. 2019; 82(1):15–26.2. Koh WJ, Moon SM, Kim SY, Woo MA, Kim S, Jhun BW, et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J. 2017; 50(3):1602503. PMID: 28954780.3. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017; 49(3):1600537. PMID: 28275170.4. Griffith DE, Aksamit TR. Managing Mycobacterium avium complex lung disease with a little help from my friend. Chest. 2021; 159(4):1372–1381. PMID: 33080299.5. Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020; 56(1):2000535. PMID: 32636299.6. Park SW, Song JW, Shim TS, Park MS, Lee HL, Uh ST, et al. Mycobacterial pulmonary infections in patients with idiopathic pulmonary fibrosis. J Korean Med Sci. 2012; 27(8):896–900. PMID: 22876056.7. Hirama T, Brode SK, Marras TK. Radiologic types of Mycobacterium xenopi pulmonary disease: different patients with similar short-term outcomes. Eur J Clin Microbiol Infect Dis. 2019; 38(2):373–381. PMID: 30552538.8. Lim J, Lyu J, Choi CM, Oh YM, Lee SD, Kim WS, et al. Non-tuberculous mycobacterial diseases presenting as solitary pulmonary nodules. Int J Tuberc Lung Dis. 2010; 14(12):1635–1640. PMID: 21144251.9. Kwon YS, Kwon BS, Kim OH, Park YE, Shim TS, Chong YP, et al. Treatment outcomes after discontinuation of ethambutol due to adverse events in Mycobacterium avium complex lung disease. J Korean Med Sci. 2020; 35(9):e59. PMID: 32141249.10. Kwon YS, Han M, Kwon BS, Kim OH, Lee HY, Shim TS, et al. Discontinuation rates attributed to adverse events and treatment outcomes between clarithromycin and azithromycin in Mycobacterium avium complex lung disease: a propensity score analysis. J Glob Antimicrob Resist. 2020; 22:106–112. PMID: 32004723.11. Kim OH, Kwon BS, Han M, Koh Y, Kim WS, Song JW, et al. Association between duration of aminoglycoside treatment and outcome of cavitary Mycobacterium avium complex lung disease. Clin Infect Dis. 2019; 68(11):1870–1876. PMID: 30239615.12. Park YE, Lee JH, Chong YP, Lee HJ, Kim HC, Song JW, et al. Treatment outcomes of the interstitial lung disease subtype of unclassifiable type Mycobacterium avium complex pulmonary disease. J Infect Chemother. 2022; 28(8):1112–1118. PMID: 35400550.13. van Ingen J, Aksamit T, Andrejak C, Böttger EC, Cambau E, Daley CL, et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J. 2018; 51(3):1800170. PMID: 29567726.14. Jhun BW, Kim SY, Moon SM, Jeon K, Kwon OJ, Huh HJ, et al. Development of macrolide resistance and reinfection in refractory Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2018; 198(10):1322–1330. PMID: 29877739.15. Philley JV, Wallace RJ Jr, Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest. 2015; 148(2):499–506. PMID: 25675393.16. Lee JH, Park YE, Chong YP, Shim TS, Jo KW. Efficacy of fluoroquinolones as substitutes for ethambutol or rifampin in the treatment of Mycobacterium avium complex pulmonary disease according to radiologic types. Antimicrob Agents Chemother. 2022; 66(2):e0152221. PMID: 34930036.17. Moon SM, Yoo IY, Huh HJ, Lee NY, Jhun BW. Intermittent treatment with azithromycin and ethambutol for noncavitary Mycobacterium avium complex pulmonary disease. Antimicrob Agents Chemother. 2019; 64(1):e01787-19. PMID: 31611366.18. Wallace RJ Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, et al. Macrolide/Azalide therapy for nodular/bronchiectatic Mycobacterium avium complex lung disease. Chest. 2014; 146(2):276–282. PMID: 24457542.19. Chae G, Park YE, Chong YP, Lee HJ, Shim TS, Jo KW. Treatment outcomes of cavitary nodular bronchiectatic-type Mycobacterium avium complex pulmonary disease. Antimicrob Agents Chemother. 2022; 66(9):e0226121. PMID: 35950842.20. Kuroishi S, Nakamura Y, Hayakawa H, Shirai M, Nakano Y, Yasuda K, et al. Mycobacterium avium complex disease: prognostic implication of high-resolution computed tomography findings. Eur Respir J. 2008; 32(1):147–152. PMID: 18321941.21. Pan SW, Shu CC, Feng JY, Su WJ. Treatment for Mycobacterium avium complex lung disease. J Formos Med Assoc. 2020; 119(Suppl 1):S67–S75. PMID: 32446754.22. Pennington KM, Vu A, Challener D, Rivera CG, Shweta FN, Zeuli JD, et al. Approach to the diagnosis and treatment of non-tuberculous mycobacterial disease. J Clin Tuberc Other Mycobact Dis. 2021; 24:100244. PMID: 34036184.23. Kobashi Y, Fukuda M, Yoshida K, Miyashita N, Niki Y, Oka M. Four cases of pulmonary Mycobacterium avium intracellulare complex presenting as a solitary pulmonary nodule and a review of other cases in Japan. Respirology. 2006; 11(3):317–321. PMID: 16635091.24. O’Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987; 135(5):1007–1014. PMID: 3579001.25. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175(4):367–416. PMID: 17277290.26. Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee JH, Zhang Y, et al. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003; 167(6):828–834. PMID: 12433668.27. Simner PJ, Woods GL, Wengenack NL. Mycobacteria. Microbiol Spectr. 2016; 4(4):28. Fujita J, Ohtsuki Y, Suemitsu I, Shigeto E, Yamadori I, Obayashi Y, et al. Pathological and radiological changes in resected lung specimens in Mycobacterium avium intracellulare complex disease. Eur Respir J. 1999; 13(3):535–540. PMID: 10232422.29. Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis. 2016; 45:123–134. PMID: 26976549.30. Obayashi Y, Fujita J, Suemitsu I, Kamei T, Nii M, Takahara J. Successive follow-up of chest computed tomography in patients with Mycobacterium avium-intracellulare complex. Respir Med. 1999; 93(1):11–15. PMID: 10464842.31. Miura K, Nakamura M, Taooka Y, Hotta T, Hamaguchi M, Okimoto T, et al. Comparison of the chest computed tomography findings between patients with pulmonary tuberculosis and those with Mycobacterium avium complex lung disease. Respir Investig. 2020; 58(3):137–143.32. Miller WT Jr, Panosian JS. Causes and imaging patterns of tree-in-bud opacities. Chest. 2013; 144(6):1883–1892. PMID: 23948769.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rapid identification of mycobacterium avium and mycobacterium intracellulare by the amplification of rRNA sequences
- Acute pneumonia caused by mycobacterium intracellulare
- A Case of Pulmonary and Endobronchial Mycobacterium avium Infection Presenting as an Acute Pneumonia in an Immunocompetent Patient
- Diagnosis and Treatment of Nontuberculous Mycobacterial Lung Disease
- Vertebral Osteomyelitis due to Mycobacterium intracellulare in an Immunocompetent Elderly Patient After Vertebroplasty