Cancer Res Treat.
2023 Jan;55(1):179-188. 10.4143/crt.2021.1512.
Universal Screening for Lynch Syndrome Compared with Pedigree-Based Screening: 10-Year Experience in a Tertiary Hospital
- Affiliations
-
- 1Department of Surgery, Uijeongbu Eulji Medical Center, Uijeongbu, Korea
- 2Department of Surgery, Seoul National University Bundang Hospital, Seongnam, Korea
- 3Department of Pathology, Seoul National University Hospital, Seoul, Korea
- 4Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2538003
- DOI: http://doi.org/10.4143/crt.2021.1512
Abstract
- Purpose
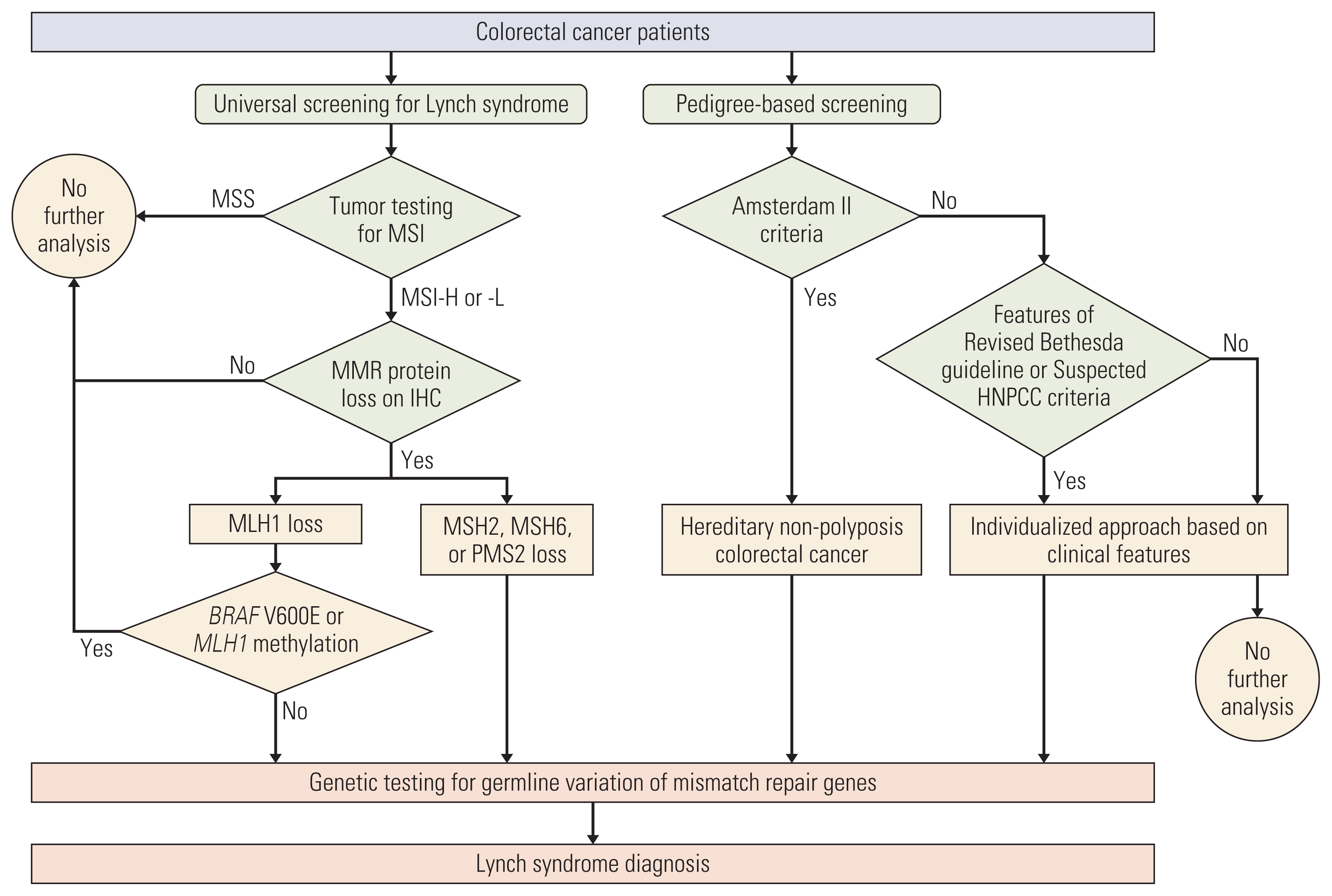
Universal screening for Lynch syndrome (LS) refers to routine tumor testing for microsatellite instability (MSI) among all patients with colorectal cancer (CRC). Despite its widespread adoption, real-world data on the yield is lacking in Korean population. We studied the yield of adopting universal screening for LS in comparison with pedigree-based screening in a tertiary center.
Materials and Methods
CRC patients from 2007-2018 were reviewed. Family histories were obtained and were evaluated for hereditary nonpolyposis colorectal cancer (HNPCC) using Amsterdam II criteria. Tumor testing for MSI began in 2007 and genetic testing was offered using all available clinicopathologic data. Yield of genetic testing for LS was compared for each approach and step.
Results
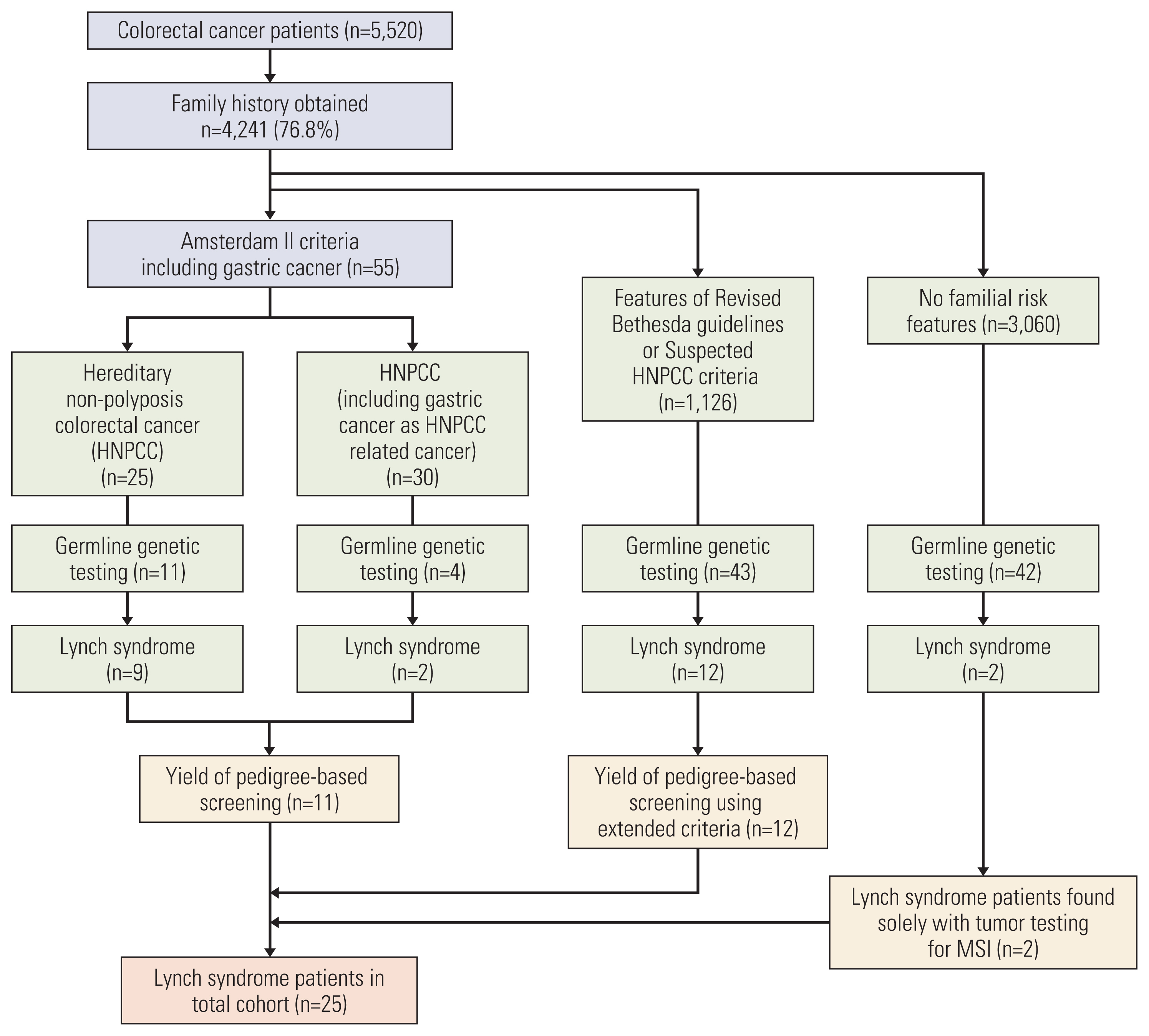
Of the 5,520 patients, tumor testing was performed in 4,701 patients (85.2%) and family histories were obtained from 4,241 patients (76.8%). Hereditary CRC (LS or HNPCC) was present in 69 patients (1.3%). MSI-high was present in 6.9%, and 25 patients had confirmed LS. Genetic testing was performed in 41.2% (47/114) of MSI-high patients, out of which 40.4% (19/47) were diagnosed with LS. There were six additional LS patients found outside of tumor testing. For pedigree-based screening, Amsterdam II criteria diagnosed 55 patients with HNPCC. Fifteen of these patients underwent genetic testing, and 11 (73.3%) were diagnosed with LS. Two patients without prior family history were diagnosed with LS and relied solely on tumor testing results.
Conclusion
Despite widespread adoption of routine tumor testing for MSI, this is not a fail-safe approach to screen all LS patients. Obtaining a thorough family history in combination with universal screening provides a more comprehensive ‘universal’ screening method for LS.
Keyword
Figure
Reference
-
References
1. Jung KW, Won YJ, Hong S, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in Korea, 2020. Cancer Res Treat. 2020; 52:351–8.2. Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer: analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000; 343:78–85.3. Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005; 352:1851–60.4. Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008; 26:5783–8.5. Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015; 110:223–62.6. Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015; 15:181–94.7. Snowsill T, Huxley N, Hoyle M, Jones-Hughes T, Coelho H, Cooper C, et al. A systematic review and economic evaluation of diagnostic strategies for Lynch syndrome. Health Technol Assess. 2014; 18:1–406.8. Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2014; 147:502–26.9. Rubenstein JH, Enns R, Heidelbaugh J, Barkun A; Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on the diagnosis and management of Lynch syndrome. Gastroenterology. 2015; 149:777–82.10. NCCN Clinical Practice Guidelines in Oncology: genetic/familial high-risk assessment: colorectal (version 2. 2019 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2019. [cited 2020 May 1]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf .11. Son IT, Kim DW, Jeong SY, Shin YK, Ihn MH, Oh HK, et al. Clinicopathological features and type of surgery for Lynch syndrome: changes during the past two decades. Cancer Res Treat. 2016; 48:605–11.12. Parsons MT, Buchanan DD, Thompson B, Young JP, Spurdle AB. Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet. 2012; 49:151–7.13. Oh JR, Kim DW, Lee HS, Lee HE, Lee SM, Jang JH, et al. Microsatellite instability testing in Korean patients with colorectal cancer. Fam Cancer. 2012; 11:459–66.14. Lee KS, Kwak Y, Ahn S, Shin E, Oh HK, Kim DW, et al. Prognostic implication of CD274 (PD-L1) protein expression in tumor-infiltrating immune cells for microsatellite unstable and stable colorectal cancer. Cancer Immunol Immunother. 2017; 66:927–39.15. Heo Y, Kim MH, Kim DW, Lee SA, Bang S, Kim MJ, et al. Extent of pedigree required to screen for and diagnose hereditary nonpolyposis colorectal cancer: comparison of simplified and extended pedigrees. Dis Colon Rectum. 2020; 63:152–9.16. Vasen HF. Clinical description of the Lynch syndrome [hereditary nonpolyposis colorectal cancer (HNPCC)]. Fam Cancer. 2005; 4:219–25.17. Park JG, Park YJ, Wijnen JT, Vasen HF. Gene-environment interaction in hereditary nonpolyposis colorectal cancer with implications for diagnosis and genetic testing. Int J Cancer. 1999; 82:516–9.18. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004; 96:261–8.19. Park JG, Vasen HF, Park YJ, Park KJ, Peltomaki P, de Leon MP, et al. Suspected HNPCC and Amsterdam criteria II: evaluation of mutation detection rate, an international collaborative study. Int J Colorectal Dis. 2002; 17:109–14.20. Ghanipour L, Jirstrom K, Sundstrom M, Glimelius B, Birgisson H. Associations of defect mismatch repair genes with prognosis and heredity in sporadic colorectal cancer. Eur J Surg Oncol. 2017; 43:311–21.21. Choi KS, Jun JK, Lee HY, Hahm MI, Oh JH, Park EC. Increasing uptake of colorectal cancer screening in Korea: a population-based study. BMC Public Health. 2010; 10:265.22. Wei W, Liu L, Chen J, Jin K, Jiang F, Liu F, et al. Racial differences in MLH1 and MSH2 mutation: an analysis of yellow race and white race based on the InSiGHT database. J Bioinform Comput Biol. 2010; 8(Suppl 1):111–25.23. Park KJ, Chang DK, Kim HC, Kim JW. Germline variants in MLH1, MSH2, and MSH6 in Korean patients with Lynch syndrome. Lab Med Online. 2018; 8:156–66.24. Wullenweber HP, Sutter C, Autschbach F, Willeke F, Kienle P, Benner A, et al. Evaluation of Bethesda guidelines in relation to microsatellite instability. Dis Colon Rectum. 2001; 44:1281–9.25. Syngal S, Fox EA, Eng C, Kolodner RD, Garber JE. Sensitivity and specificity of clinical criteria for hereditary non-polyposis colorectal cancer associated mutations in MSH2 and MLH1. J Med Genet. 2000; 37:641–5.26. Perez-Carbonell L, Ruiz-Ponte C, Guarinos C, Alenda C, Paya A, Brea A, et al. Comparison between universal molecular screening for Lynch syndrome and revised Bethesda guidelines in a large population-based cohort of patients with colorectal cancer. Gut. 2012; 61:865–72.27. Bartley AN, Luthra R, Saraiya DS, Urbauer DL, Broaddus RR. Identification of cancer patients with Lynch syndrome: clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev Res (Phila). 2012; 5:320–7.28. Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn. 2008; 10:293–300.29. Shin YK, Heo SC, Shin JH, Hong SH, Ku JL, Yoo BC, et al. Germline mutations in MLH1, MSH2 and MSH6 in Korean hereditary non-polyposis colorectal cancer families. Hum Mutat. 2004; 24:351.30. Kastrinos F, Uno H, Ukaegbu C, Alvero C, McFarland A, Yurgelun MB, et al. Development and validation of the PRE-MM5 model for comprehensive risk assessment of Lynch syndrome. J Clin Oncol. 2017; 35:2165–72.31. Heald B, Plesec T, Liu X, Pai R, Patil D, Moline J, et al. Implementation of universal microsatellite instability and immunohistochemistry screening for diagnosing lynch syndrome in a large academic medical center. J Clin Oncol. 2013; 31:1336–40.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Screening for Lynch syndrome using risk assessment criteria in patients with ovarian cancer
- Universal Screening of Severe Acute Respiratory Syndrome Coronavirus 2 with Polymerase Chain Reaction Testing after Rally of Trainee Doctors
- Hereditary Colon Cancer: Lynch Syndrome
- A simplified two-marker immunohistochemistry strategy for Lynch syndrome screening in endometrial cancer patients
- Completeness of pedigree and family cancer history for ovarian cancer patients