Blood Res.
2022 Dec;57(4):290-293. 10.5045/br.2022.2022099.
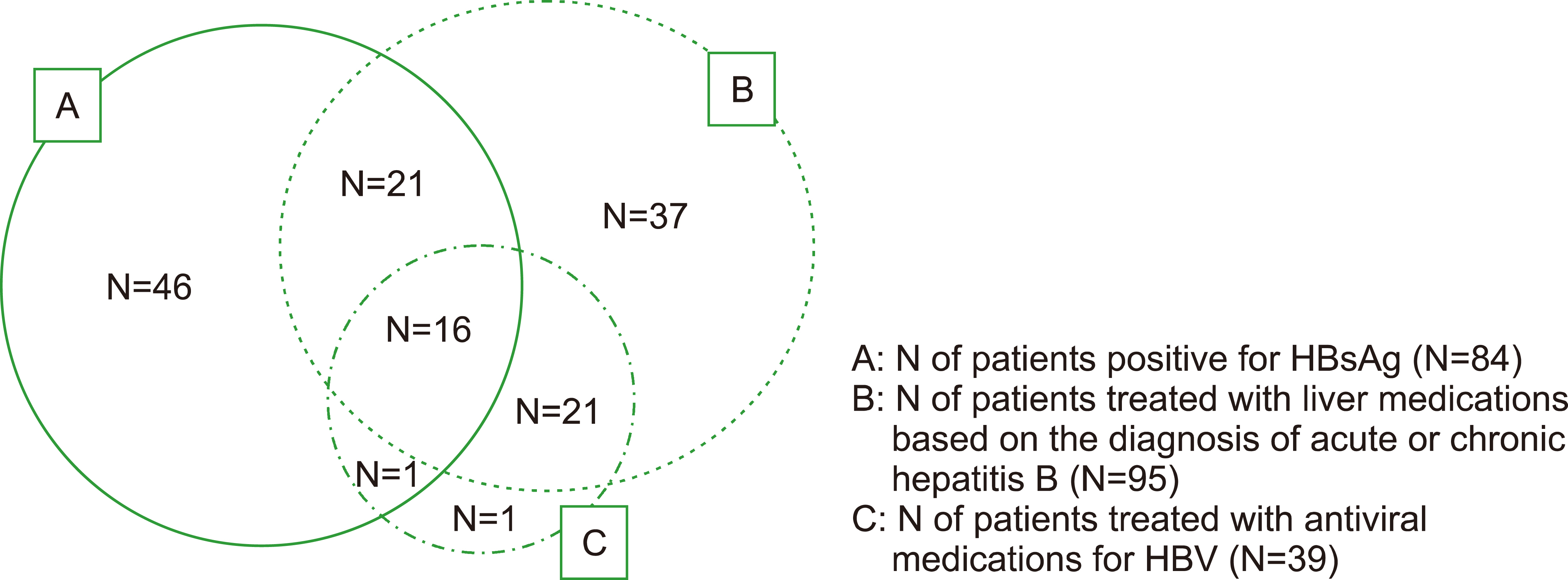
High rate of hepatitis B reactivation during tyrosine kinase inhibitor treatment among patients with chronic myeloid leukemia in Korea
- Affiliations
-
- 1Department of Hematology-Oncology, Division of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Center of Evidence-Based Medicine, Institute of Convergence Science, Yonsei University, Seoul, Korea.
- 3Center of Biomedical Data Science, Wonju, Korea.
- 4Department of Gastroenterology and Hepatology, Division of Internal Medicine, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
- KMID: 2537554
- DOI: http://doi.org/10.5045/br.2022.2022099
Figure
Reference
-
1. World Health Organization. 2019. Hepatitis B. WHO;Geneva, Switzerland: at https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed April 1, 2020.2. Cho EJ, Kim SE, Suk KT, et al. 2017; Current status and strategies for hepatitis B control in Korea. Clin Mol Hepatol. 23:205–11. DOI: 10.3350/cmh.2017.0104. PMID: 28942624. PMCID: PMC5628005.3. European Association for the Study of the Liver. 2017; EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 67:370–98. DOI: 10.1016/j.jhep.2017.03.021. PMID: 28427875.4. Terrault NA, Lok ASF, McMahon BJ, et al. 2018; Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 67:1560–99. DOI: 10.1002/hep.29800. PMID: 29405329. PMCID: PMC5975958.5. Hochhaus A, Baccarani M, Silver RT, et al. 2020; European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 34:966–84. DOI: 10.1038/s41375-020-0776-2. PMID: 32127639. PMCID: PMC7214240.6. Ikeda K, Shiga Y, Takahashi A, et al. 2006; Fatal hepatitis B virus reactivation in a chronic myeloid leukemia patient during imatinib mesylate treatment. Leuk Lymphoma. 47:155–7. DOI: 10.1080/14639230500236818. PMID: 16321842.7. Kang BW, Lee SJ, Moon JH, et al. 2009; Chronic myeloid leukemia patient manifesting fatal hepatitis B virus reactivation during treatment with imatinib rescued by liver transplantation: case report and literature review. Int J Hematol. 90:383–7. DOI: 10.1007/s12185-009-0386-2. PMID: 19641858.8. Lai GM, Yan SL, Chang CS, Tsai CY. 2013; Hepatitis B reactivation in chronic myeloid leukemia patients receiving tyrosine kinase inhibitor. World J Gastroenterol. 19:1318–21. DOI: 10.3748/wjg.v19.i8.1318. PMID: 23483799. PMCID: PMC3587491.9. Ando T, Kojima K, Isoda H, et al. 2015; Reactivation of resolved infection with the hepatitis B virus immune escape mutant G145R during dasatinib treatment for chronic myeloid leukemia. Int J Hematol. 102:379–82. DOI: 10.1007/s12185-015-1788-y. PMID: 25842192.10. Temel T, Gunduz E, Sadigova E, Uskudar Teke H, Meric Ozgenel S, Harmanci Ozakyol A. 2015; Hepatitis B virus reactivation under treatment with nilotinib. Euroasian J Hepatogastroenterol. 5:112–4. DOI: 10.5005/jp-journals-10018-1147. PMID: 29201705. PMCID: PMC5578539.11. Orlandi EM, Elena C, Bono E. 2017; Risk of hepatitis B reactivation under treatment with tyrosine-kinase inhibitors for chronic myeloid leukemia. Leuk Lymphoma. 58:1764–6. DOI: 10.1080/10428194.2016.1260127. PMID: 27892750.12. Wang YH, Liang JD, Sheng WH, Tien FM, Chen CY, Tien HF. 2019; Hepatitis B reactivation during treatment of tyrosine kinase inhibitors-experience in 142 adult patients with chronic myeloid leukemia. Leuk Res. 81:95–7. DOI: 10.1016/j.leukres.2019.05.001. PMID: 31075669.13. Korean Association for the Study of the Liver (KASL). 2019; KASL clinical practice guidelines for management of chronic hepatitis B. Clin Mol Hepatol. 25:93–159. DOI: 10.3350/cmh.2019.1002. PMID: 31185710. PMCID: PMC6589848.14. Health Insurance Review & Assessment Service. 2020. Drug Classification Codes. HIRA;Wonju, Korea: at https://opendata.hira.or.kr/op/opc/selectMeftDivList.do. Accessed April 1, 2020.15. Sorà F, Ponziani FR, Laurenti L, et al. 2017; Low risk of hepatitis B virus reactivation in patients with resolved infection and chronic myeloid leukemia treated with tyrosine kinase inhibitors. Leuk Lymphoma. 58:993–5. DOI: 10.1080/10428194.2016.1219906. PMID: 27546591.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment-free remission after discontinuation of imatinib, dasatinib, and nilotinib in patients with chronic myeloid leukemia
- Treatment after failure of frontline therapy of chronic myeloid leukemia in chronic phase including allogeneic hematopoietic stem cell transplantation
- Development of Tyrosine Kinase Inhibitor in Chronic Myeloid Leukemia
- Chronic Myeloid Leukemia Following Radioactive Iodine Treatment for Thyroid Cancer: Two Case Reports and a Literature Review
- A Pilot with Chronic Myeloid Leukemia: Aeromedical Assessment