Korean J Physiol Pharmacol.
2023 Jan;27(1):61-73. 10.4196/kjpp.2023.27.1.61.
Exosome-mediated lnc-ABCA12-3 promotes proliferation and glycolysis but inhibits apoptosis by regulating the tolllike receptor 4/nuclear factor kappa-B signaling pathway in esophageal squamous cell carcinoma
- Affiliations
-
- 1Department of Thoracic Surgery, Affiliated Hospital of Zunyi Medical University, China
- 2Department of Cardiovascular Surgery, Affiliated Hospital of Zuinyi Medical University, Zunyi, Guizhou 563003, China
- 3The Second Department of Thoracic Surgery, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan 410031, China
- KMID: 2537504
- DOI: http://doi.org/10.4196/kjpp.2023.27.1.61
Abstract
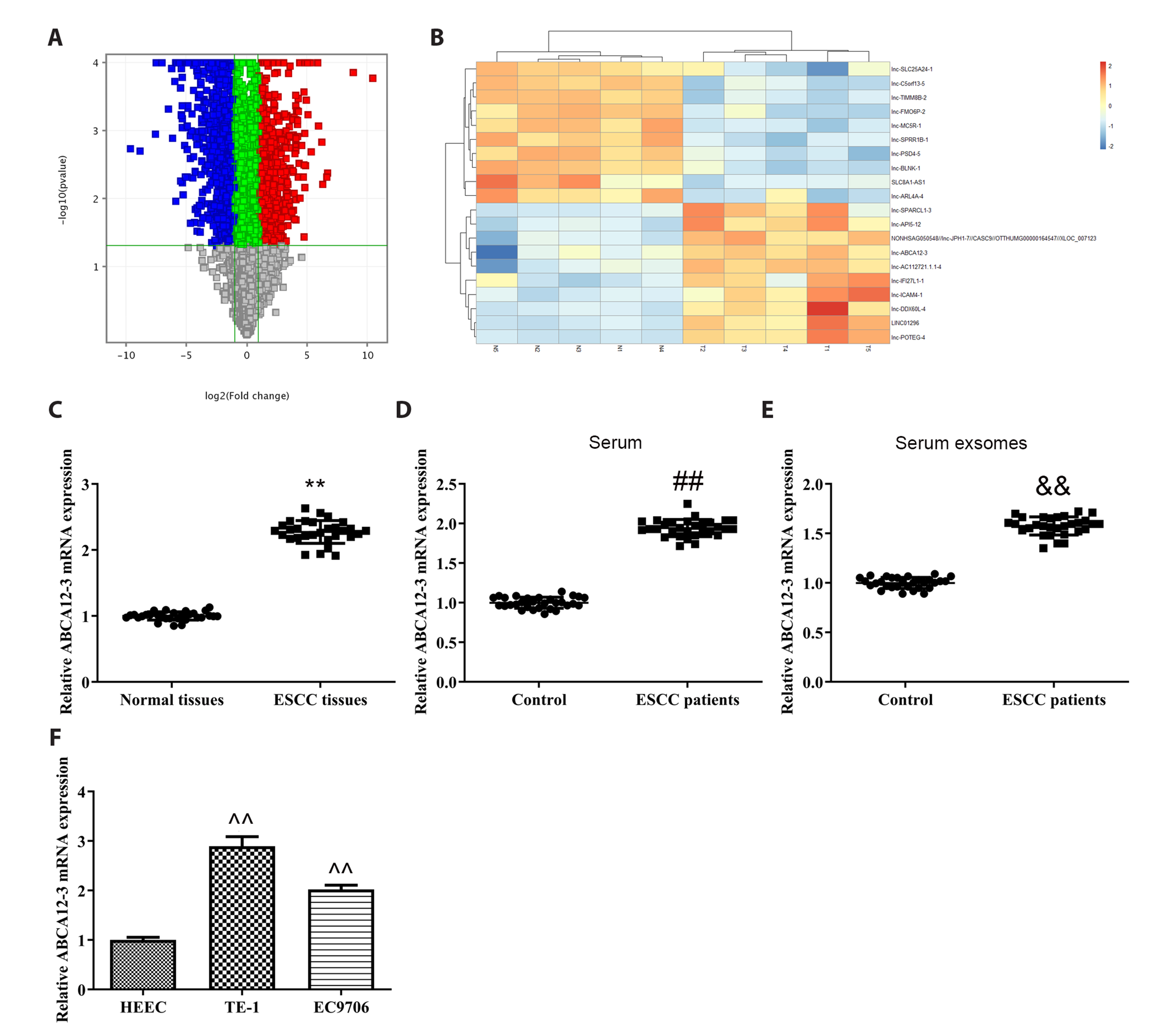
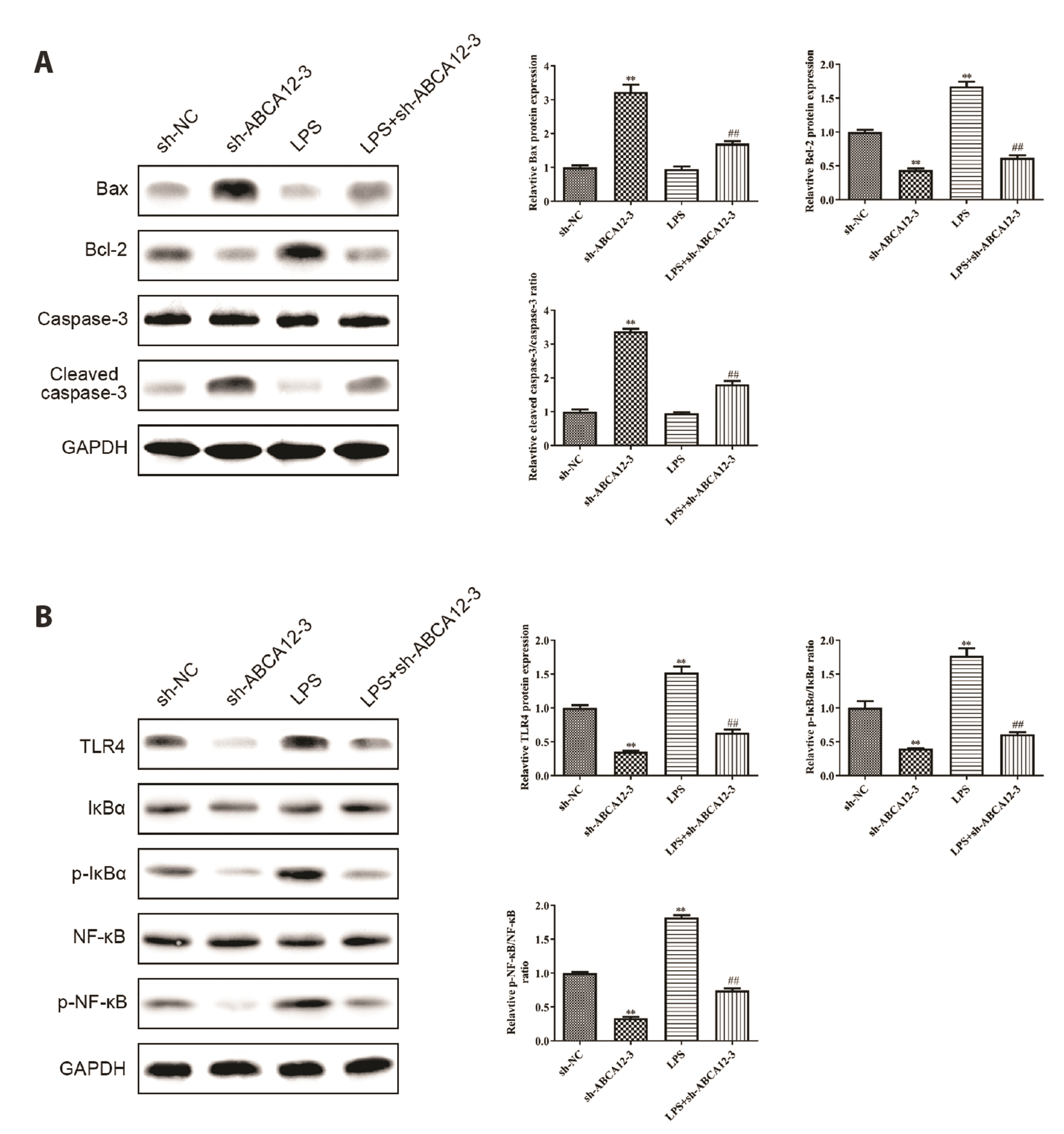
- Esophageal squamous cell carcinoma (ESCC) is a kind of malignant tumor with high incidence and mortality in the digestive system. The aim of this study is to explore the function of lnc-ABCA12-3 in the development of ESCC and its unique mechanisms. RT-PCR was applied to detect gene transcription levels in tissues or cell lines like TE-1, EC9706, and HEEC cells. Western blot was conducted to identify protein expression levels of mitochondrial apoptosis and toll-like receptor 4 (TLR4)/nuclear factor kappa-B (NF-κB) signaling pathway. CCK-8 and EdU assays were carried out to measure cell proliferation, and cell apoptosis was examined by flow cytometry. ELISA was used for checking the changes in glycolysis-related indicators. Lnc-ABCA12-3 was highly expressed in ESCC tissues and cells, which preferred it to be a candidate target. The TE-1 and EC9706 cells proliferation and glycolysis were obviously inhibited with the downregulation of lnc-ABCA12-3, while apoptosis was promoted. TLR4 activator could largely reverse the apoptosis acceleration and relieved the proliferation and glycolysis suppression caused by lnc-ABCA12-3 downregulation. Moreover, the effect of lnc-ABCA12-3 on ESCC cells was actualized by activating the TLR4/NF-κB signaling pathway under the mediation of exosome. Taken together, the lnc-ABCA12-3 could promote the proliferation and glycolysis of ESCC, while repressing its apoptosis probably by regulating the TLR4/NF-κB signaling pathway under the mediation of exosome.
Keyword
Figure
Reference
-
1. Tang L, Zhao S, Liu W, Parchim NF, Huang J, Tang Y, Gan P, Zhong M. 2013; Diagnostic accuracy of circulating tumor cells detection in gastric cancer: systematic review and meta-analysis. BMC Cancer. 13:314. DOI: 10.1186/1471-2407-13-314. PMID: 23806209. PMCID: PMC3699416. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84879457033&origin=inward.2. Qi YJ, Chao WX, Chiu JF. 2012; An overview of esophageal squamous cell carcinoma proteomics. J Proteomics. 75:3129–3137. DOI: 10.1016/j.jprot.2012.04.025. PMID: 22564818. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84861668900&origin=inward.3. Hirano H, Kato K. 2019; Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J Clin Oncol. 49:412–420. DOI: 10.1093/jjco/hyz034. PMID: 30920626. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85067627904&origin=inward.4. Zhang R, Lau LHS, Wu PIC, Yip HC, Wong SH. Lam AK, editor. 2020. Endoscopic diagnosis and treatment of esophageal squamous cell carcinoma. Esophageal squamous cell carcinoma: Methods in molecular biology. Humana;New York: p. 47–62. https://link.springer.com/protocol/10.1007/978-1-0716-0377-2_5. DOI: 10.1007/978-1-0716-0377-2_5. PMID: 32056169. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85079361603&origin=inward.5. Fatehi Hassanabad A, Chehade R, Breadner D, Raphael J. 2020; Esophageal carcinoma: towards targeted therapies. Cell Oncol (Dordr). 43:195–209. DOI: 10.1007/s13402-019-00488-2. PMID: 31848929. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85076751284&origin=inward.6. Mathivanan S, Ji H, Simpson RJ. 2010; Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 73:1907–1920. DOI: 10.1016/j.jprot.2010.06.006. PMID: 20601276. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=77956404647&origin=inward.7. Li M, Jia J, Li S, Cui B, Huang J, Guo Z, Ma K, Wang L, Cui C. 2021; Exosomes derived from tendon stem cells promote cell proliferation and migration through the TGF β signal pathway. Biochem Biophys Res Commun. 536:88–94. DOI: 10.1016/j.bbrc.2020.12.057. PMID: 33370718. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85098153715&origin=inward.8. Lee Y, El Andaloussi S, Wood MJ. 2012; Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 21:R125–R134. DOI: 10.1093/hmg/dds317. PMID: 22872698. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84867118648&origin=inward.9. Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, Coffey RJ. 2011; Amphiregulin exosomes increase cancer cell invasion. Curr Biol. 21:779–786. DOI: 10.1016/j.cub.2011.03.043. PMID: 21514161. PMCID: PMC3417320. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=79955652381&origin=inward.10. Iwanicki-Caron I, Basile P, Toure E, Antonietti M, Lecleire S, Di Fiore A, Oden-Gangloff A, Blanchard F, Lemoine F, Di Fiore F, Sabourin JC, Michel P. 2013; Usefulness of circulating tumor cell detection in pancreatic adenocarcinoma diagnosis. Am J Gastroenterol. 108:152–155. DOI: 10.1038/ajg.2012.367. PMID: 23287955. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84872040710&origin=inward.11. Yang G, Lu X, Yuan L. 2014; LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 1839:1097–1109. DOI: 10.1016/j.bbagrm.2014.08.012. PMID: 25159663. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84907500489&origin=inward.12. Li RQ, Ren Y, Liu W, Pan W, Xu FJ, Yang M. 2017; MicroRNA-mediated silence of onco-lncRNA MALAT1 in different ESCC cells via ligand-functionalized hydroxyl-rich nanovectors. Nanoscale. 9:2521–2530. DOI: 10.1039/C6NR09668A. PMID: 28150831. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85013175483&origin=inward.13. Zang B, Zhao J, Chen C. 2019; LncRNA PCAT-1 promoted ESCC progression via regulating ANXA10 expression by sponging miR-508-3p. Cancer Manag Res. 11:10841–10849. DOI: 10.2147/CMAR.S233983. PMID: 31920393. PMCID: PMC6941610. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85078555094&origin=inward.14. Tang Y, Yang P, Zhu Y, Su Y. 2020; LncRNA TUG1 contributes to ESCC progression via regulating miR-148a-3p/MCL-1/Wnt/β-catenin axis in vitro. Thorac Cancer. 11:82–94. DOI: 10.1111/1759-7714.13236. PMID: 31742924. PMCID: PMC6938768. PMID: dff3726bac2d4c4d8038f766b925d4ff. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85075121104&origin=inward.15. Yi S, Zhang W, Zhou Q, Cheng G. 2018; lncRNA XIST promotes aggressive tumor phenotype and is associated with poor prognosis in esophageal squamous cell carcinoma. Int J Clin Exp Med. 11:8317–8323. https://e-century.us/files/ijcem/11/8/ijcem0070409.pdf.16. Zhang L, Li P, Xing C, Zhu D, Zhang J, Wang W, Jiang G. 2020. A multi-lncRNA signature for suvival prediction in esophageal squamous cell carcinoma (ESCC) patients. Research Square. [Preprint]. Available from: https://doi.org/10.21203/rs.3.rs-26795/v1. November 2022. DOI: 10.21203/rs.3.rs-26795/v1.17. Ma J, Xiao Y, Tian B, Chen S, Zhang B, Wu J, Wu Z, Li X, Tang J, Yang D, Zhou Y, Wang H, Su M, Wang W. 2020; Long noncoding RNA lnc-ABCA12-3 promotes cell migration, invasion, and proliferation by regulating fibronectin 1 in esophageal squamous cell carcinoma. J Cell Biochem. 121:1374–1387. DOI: 10.1002/jcb.29373. PMID: 31512786. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85073792063&origin=inward.18. Yuan B, Liu D, Liu X. 2014; Spinal cord stimulation exerts analgesia effects in chronic constriction injury rats via suppression of the TLR4/NF-κB pathway. Neurosci Lett. 581:63–68. DOI: 10.1016/j.neulet.2014.08.023. PMID: 25153517. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84906736583&origin=inward.19. Wang B, Li J, Tian F. 2021; Downregulation of lncRNA SNHG14 attenuates osteoarthritis by inhibiting FSTL-1 mediated NLRP3 and TLR4/NF-κB pathway through miR-124-3p. Life Sci. 270:119143. DOI: 10.1016/j.lfs.2021.119143. PMID: 33539913. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85100261845&origin=inward.20. Li X, Li H, Dong X, Wang X, Zhu J, Cheng Y, Fan P. 2018; Expression of NF-κB and TLR-4 is associated with the occurrence, progression and prognosis of esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 11:5850–5859. PMID: 31949671. PMCID: PMC6963060.21. Jiao LI, Jingjing MA, Xue HU, Dong W. Department of Gastroenterology, Renmin Hospital of Wuhan University. 2018; The study of α-hederin induces the apoptosis of esophageal carcinoma cells via the mitochondrial pathway mediated by reactive oxygen species. Chin J Diffic Complicat Cases. 017:932–935. https://xueshu.baidu.com/usercenter/paper/show?paperid=1r3c0g702w410aw0yt0u0p104u751516&site=xueshu_se&hitarticle=1.22. Wu B, Li J, Ni H, Zhuang X, Qi Z, Chen Q, Wen Z, Shi H, Luo X, Jin B. 2018; TLR4 activation promotes the progression of experimental autoimmune myocarditis to dilated cardiomyopathy by inducing mitochondrial dynamic imbalance. Oxid Med Cell Longev. 2018:3181278. DOI: 10.1155/2018/3181278. PMID: 30046376. PMCID: PMC6038665. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85055033580&origin=inward.23. Huang CY, Lee CH, Tu CC, Wu CH, Huang MT, Wei PL, Chang YJ. 2018; Glucose-regulated protein 94 mediates progression and metastasis of esophageal squamous cell carcinoma via mitochondrial function and the NF-kB/COX-2/VEGF axis. Oncotarget. 9:9425–9441. DOI: 10.18632/oncotarget.24114. PMID: 29507700. PMCID: PMC5823643. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85041390521&origin=inward.24. Madden J, Barrett C, Laffir FR, Thompson M, Galvin P, O' Riordan A. 2021; On-chip glucose detection based on glucose oxidase immobilized on a platinum-modified, gold microband electrode. Biosensors (Basel). 11:249. DOI: 10.3390/bios11080249. PMID: 34436051. PMCID: PMC8392376. PMID: 2c82941c02e144deb3d5206656d4bbe9. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85111928543&origin=inward.25. Fisher C, Christison T, Verma M, Yang H. 2014. Rapid determination of lactose and lactulose in dairy products using high-performance anion-exchange chromatography with pulsed amperometric detection. Thermo Scientific;Sunnyvale: p. 1–7. http://www.thermoscientific.com/content/dam/tfs/ATG/CMD/cmd-documents/sci-res/posters/ms/events/AOAC-2014/PN-71295-AOAC2014-Lactose-Lactulose-Dairy-PN71295-EN.pdf.26. Zhang X, Liang W, Liu J, Zang X, Gu J, Pan L, Shi H, Fu M, Huang Z, Zhang Y, Qian H, Jiang P, Xu W. 2018; Long non-coding RNA UFC1 promotes gastric cancer progression by regulating miR-498/Lin28b. J Exp Clin Cancer Res. 37:134. DOI: 10.1186/s13046-018-0803-6. PMID: 29970131. PMCID: PMC6029056. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85049439127&origin=inward.27. Wang L, Zhu R, Huang Z, Li H, Zhu H. 2013; Lipopolysaccharide-induced toll-like receptor 4 signaling in cancer cells promotes cell survival and proliferation in hepatocellular carcinoma. Dig Dis Sci. 58:2223–2236. DOI: 10.1007/s10620-013-2745-3. PMID: 23828139. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84881481381&origin=inward.28. Kunizaki M, Hamasaki K, Wakata K, Tobinaga S, Sumida Y, Hidaka S, Yasutake T, Miyazaki T, Matsumoto K, Yamasaki T, Sawai T, Hamamoto R, Nanashima A, Nagayasu T. 2018; Clinical value of serum p53 antibody in the diagnosis and prognosis of esophageal squamous cell carcinoma. Anticancer Res. 38:1807–1813. DOI: 10.21873/anticanres.12419. PMID: 29491120. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85043437825&origin=inward.29. Xie H, Ma H, Zhou D. 2013; Plasma HULC as a promising novel biomarker for the detection of hepatocellular carcinoma. Biomed Res Int. 2013:136106. DOI: 10.1155/2013/136106. PMID: 23762823. PMCID: PMC3674644. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84878692521&origin=inward.30. Ifere GO, Ananaba GA. 2009; Prostate cancer gene expression marker 1 (PCGEM1): a patented prostate- specific non-coding gene and regulator of prostate cancer progression. Recent Pat DNA Gene Seq. 3:151–163. DOI: 10.2174/187221509789318360. PMID: 19891595. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70449562559&origin=inward.31. Zhang S, Liang Y, Wu Y, Chen X, Wang K, Li J, Guan X, Xiong G, Yang K, Bai Y. 2019; Upregulation of a novel lncRNA LINC01980 promotes tumor growth of esophageal squamous cell carcinoma. Biochem Biophys Res Commun. 513:73–80. DOI: 10.1016/j.bbrc.2019.03.012. PMID: 30935686. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064477662&origin=inward.32. Wu Y, Hu L, Liang Y, Li J, Wang K, Chen X, Meng H, Guan X, Yang K, Bai Y. 2017; Up-regulation of lncRNA CASC9 promotes esophageal squamous cell carcinoma growth by negatively regulating PDCD4 expression through EZH2. Mol Cancer. 16:150. DOI: 10.1186/s12943-017-0715-7. PMID: 28854977. PMCID: PMC5577767. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85028501375&origin=inward.33. Kang M, Ren M, Li Y, Fu Y, Deng M, Li C. 2018; Exosome-mediated transfer of lncRNA PART1 induces gefitinib resistance in esophageal squamous cell carcinoma via functioning as a competing endogenous RNA. J Exp Clin Cancer Res. 37:171. DOI: 10.1186/s13046-018-0845-9. PMCID: PMC6063009. PMID: 30049286. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85050655985&origin=inward.34. Yu Y, Pang D, Li C, Gu X, Chen Y, Ou R, Wei Q, Shang H. 2022; The expression discrepancy and characteristics of long non-coding RNAs in peripheral blood leukocytes from amyotrophic lateral sclerosis patients. Mol Neurobiol. 59:3678–3689. DOI: 10.1007/s12035-022-02789-4. PMID: 35364800. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85127377431&origin=inward.35. Zang W, Wang T, Huang J, Li M, Wang Y, Du Y, Chen X, Zhao G. 2015; Long noncoding RNA PEG10 regulates proliferation and invasion of esophageal cancer cells. Cancer Gene Ther. 22:138–144. DOI: 10.1038/cgt.2014.77. PMID: 25591808. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84927695718&origin=inward.36. Wang L, Zhang Z, Wang H. 2021; Downregulation of lncRNA GAS5 prevents mitochondrial apoptosis and hypoxic-ischemic brain damage in neonatal rats through the microRNA-128-3p/Bax/Akt/GSK-3β axis. Neuroreport. 32:1395–1402. DOI: 10.1097/WNR.0000000000001730. PMID: 34718247. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85121388547&origin=inward.37. Luo G, Wang M, Wu X, Tao D, Xiao X, Wang L, Min F, Zeng F, Jiang G. 2015; Long non-coding RNA MEG3 inhibits cell proliferation and induces apoptosis in prostate cancer. Cell Physiol Biochem. 37:2209–2220. DOI: 10.1159/000438577. PMID: 26610246. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84948654427&origin=inward.38. Wang W, Luo P, Guo W, Shi Y, Xu D, Zheng H, Jia L. 2018; LncRNA SNHG20 knockdown suppresses the osteosarcoma tumorigenesis through the mitochondrial apoptosis pathway by miR-139/RUNX2 axis. Biochem Biophys Res Commun. 503:1927–1933. DOI: 10.1016/j.bbrc.2018.07.137. PMID: 30072099. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053113010&origin=inward.39. Yang L, Sun K, Chu J, Qu Y, Zhao X, Yin H, Ming L, Wan J, He F. 2018; Long non-coding RNA FTH1P3 regulated metastasis and invasion of esophageal squamous cell carcinoma through SP1/NF-kB pathway. Biomed Pharmacother. 106:1570–1577. DOI: 10.1016/j.biopha.2018.07.129. PMID: 30119232. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85050542732&origin=inward.40. Lu Z, Chen Z, Li Y, Wang J, Zhang Z, Che Y, Huang J, Sun S, Mao S, Lei Y, Gao Y, He J. 2018; TGF-β-induced NKILA inhibits ESCC cell migration and invasion through NF-κB/MMP14 signaling. J Mol Med (Berl). 96:301–313. DOI: 10.1007/s00109-018-1621-1. PMID: 29379981. PMCID: PMC5859688. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85041130871&origin=inward.41. Zu Y, Ping W, Deng T, Zhang N, Fu X, Sun W. 2017; Lipopolysaccharide-induced toll-like receptor 4 signaling in esophageal squamous cell carcinoma promotes tumor proliferation and regulates inflammatory cytokines expression. Dis Esophagus. 30:1–8. DOI: 10.1111/dote.12466. PMID: 27061118. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84963788662&origin=inward.42. Jiao Z, Yu A, Rong W, He X, Zen K, Shi M, Wang T. 2020; Five-lncRNA signature in plasma exosomes serves as diagnostic biomarker for esophageal squamous cell carcinoma. Aging (Albany NY). 12:15002–15010. DOI: 10.18632/aging.103559. PMID: 32597791. PMCID: PMC7425435. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85088820693&origin=inward.43. Wang L, Zhang J. 2020; Exosomal lncRNA AK139128 derived from hypoxic cardiomyocytes promotes apoptosis and inhibits cell proliferation in cardiac fibroblasts. Int J Nanomedicine. 15:3363–3376. DOI: 10.2147/IJN.S240660. PMID: 32494135. PMCID: PMC7229807. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084801267&origin=inward.44. Wen L, Mao W, Xu L, Cai B, Gu L. 2022; Sesamin exerts anti-tumor activity in esophageal squamous cell carcinoma via inhibition of TRIM44 and NF-κB signaling. Chem Biol Drug Des. 99:118–125. DOI: 10.1111/cbdd.13937. PMID: 34411455. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85114338082&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of cell growth and induction of apoptosis by bilobalide in FaDu human pharyngeal squamous cell carcinoma
- Antitumor effects of octyl gallate on hypopharyngeal carcinoma cells
- PLCE1 Promotes the Invasion and Migration of Esophageal Cancer Cells by Up-Regulating the PKCα/NF-κB Pathway
- NF-kappaB-Mediated Regulation of Osteoclastogenesis
- Anti-Tumor Effects of Vascular Endothelial Growth Factor Inhibitor on Oral Squamous Cell Carcinoma Cell Lines