Ann Pediatr Endocrinol Metab.
2022 Dec;27(4):308-314. 10.6065/apem.2244038.019.
Remission in pediatric Graves’ disease treated with antithyroid drug and the risk factors associated with relapse
- Affiliations
-
- 1Department of Paediatrics, Queen Elizabeth Hospital Hong Kong, Hong Kong, Hong Kong
- KMID: 2537243
- DOI: http://doi.org/10.6065/apem.2244038.019
Abstract
- Purpose
To evaluate the characteristics and frequency of remission in pediatric patients with Graves’ disease (GD) treated with antithyroid drug (ATD) and to identify factors that may be associated with relapse.
Methods
Medical records of patients younger than 19 years who presented to the Department of Pediatrics of Queen Elizabeth Hospital Hong Kong with newly diagnosed GD from 1st January 2007 to 31st December 2017 were retrospectively reviewed. Remission was defined as euthyroidism for 12 months or more after discontinuation of ATD treatment and no relapses during the follow-up period. Patients who successfully achieved remission were compared to those who suffered relapse. Factors that may predict occurrence of relapse after ATD treatments were studied, and their odds ratios (ORs) were calculated.
Results
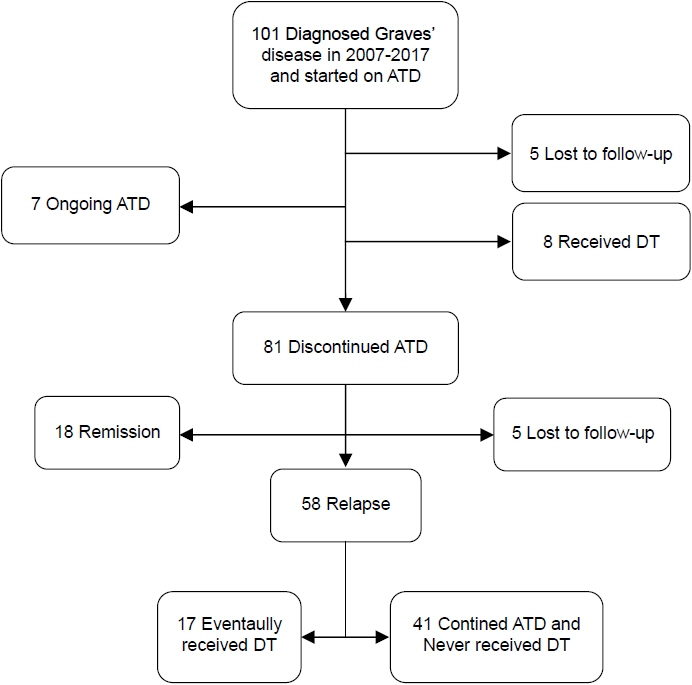
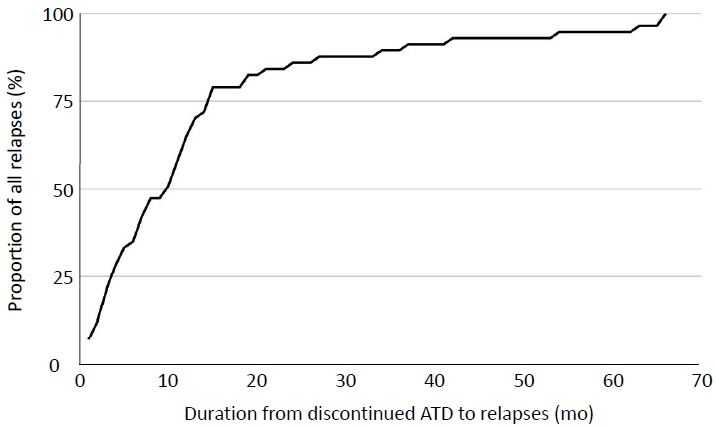
A total of 101 patients was included in this study. Eighty-one patients completed one course of ATD. Eighteen patients (17.8%) successfully achieved remission, and 58 patients (57.4%) experienced relapse after discontinuation of ATD. The remission group received a significantly longer course of ATD therapy than the relapse group (median, 28 months; interquartile range [IQR], 18–48 months in remission group vs. median, 21 months; IQR, 17–26; p=0.024). The OR for relapse was 0.971 (95% confidence interval [CI], 0.946–0.997) in univariate analysis and remained significant after adjustments in the multivariate regression model (OR, 0.961; 95% CI, 0.933–0.989; p=0.008).
Conclusion
The remission rate in pediatric patients with GD treated with ATD was low. A longer ATD course was associated with a greater chance of remission in this population.
Figure
Reference
-
References
1. Srinivasan S, Misra M. Hyperthyroidism in children. Pediatr Rev. 2015; 36:239–48.2. Wong GW, Cheng PS. Increasing incidence of childhood Graves' disease in Hong Kong: a follow-up study. Clin Endocrinol (Oxf). 2001; 54:547–50.3. Léger J, Carel JC. Management of endocrine disease: arguments for the prolonged use of antithyroid drugs in children with Graves' disease. Eur J Endocrinol. 2017; 177:R59–67.4. Mooij CF, Cheetham TD, Verburg FA, Eckstein A, Pearce SH, Léger J, et al. 2022 European Thyroid Association Guideline for the management of pediatric Graves' disease. Eur Thyroid J. 2022; 11:e210073.5. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, et al. 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016; 26:1343–421.6. Committee on Pharmaceutical Affairs; Japanese Society for Pediatric Endocrinology, and the Pediatric Thyroid Disease Committee; Japan Thyroid Association (Taskforce for the Revision of the Guidelines for the Treatment of Childhood-Onset Graves' Disease), Minamitani K, Sato H, et al. Guidelines for the treatment of childhood-onset Graves' disease in Japan, 2016. Clin Pediatr Endocrinol. 2017; 26:29–62.7. Léger J, Gelwane G, Kaguelidou F, Benmerad M, Alberti C; French Childhood Graves' Disease Study Group. Positive impact of long-term antithyroid drug treatment on the outcome of children with Graves' disease: national longterm cohort study. J Clin Endocrinol Metab. 2012; 97:110–9.8. Kaguelidou F, Alberti C, Castanet M, Guitteny MA, Czernichow P, Léger J, et al. Predictors of autoimmune hyperthyroidism relapse in children after discontinuation of antithyroid drug treatment. J Clin Endocrinol Metab. 2008; 93:3817–26.9. Ohye H, Minagawa A, Noh JY, Mukasa K, Kunii Y, Watanabe N, et al. Antithyroid drug treatment for graves' disease in children: a long-term retrospective study at a single institution. Thyroid. 2014; 24:200–7.10. Azizi F, Takyar M, Madreseh E, Amouzegar A. Longterm methimazole therapy in juvenile Graves' disease: a randomized trial. Pediatrics. 2019; 143:e20183034.11. van Lieshout JM, Mooij CF, van Trotsenburg ASP, Zwaveling-Soonawala N. Methimazole-induced remission rates in pediatric Graves' disease: a systematic review. Eur J Endocrinol. 2021; 185:219–29.12. Bayramoğlu E, Elmaogulları S, Sagsak E, Aycan Z. Evaluation of long-term follow-up and methimazole therapy outcomes of pediatric Graves' disease: a singlecenter experience. J Pediatr Endocrinol Metab. 2019; 32:341–6.13. Tunç S, Köprülü Ö, Ortaç H, Nalbantoğlu Ö, Dizdarer C, Demir K, et al. Long-term monitoring of Graves' disease in children and adolescents: a single-center experience. Turk J Med Sci. 2019; 49:464–71.14. Song A, Kim SJ, Kim MS, Kim J, Kim I, Bae GY, et al. Long-term antithyroid drug treatment of Graves' disease in children and adolescents: a 20-year single-center experience. Front Endocrinol (Lausanne). 2021; 12:687834.15. Rabon S, Burton AM, White PC. Graves' disease in children: long-term outcomes of medical therapy. Clin Endocrinol (Oxf). 2016; 85:632–5.16. Glaser NS, Styne DM. Predictors of early remission of hyperthyroidism in children. J Clin Endocrinol Metab. 1997; 82:1719–26.17. Léger J. Graves' disease in children. Endocr Dev. 2014; 26:171–82.18. Sosa JA, Tuggle CT, Wang TS, Thomas DC, Boudourakis L, Rivkees S, et al. Clinical and economic outcomes of thyroid and parathyroid surgery in children. J Clin Endocrinol Metab. 2008; 93:3058–65.19. Rivkees SA. Controversies in the management of Graves' disease in children. J Endocrinol Invest. 2016; 39:1247–57.20. Cheetham T. How to use thionamide anti-thyroid drug in the young- what's new? Thyroid Res. 2021; 14:18.21. Weetman AP, McGregor AM, Hall R. Methimazole inhibits thyroid autoantibody production by an action on accessory cells. Clin Immunol Immunopathol. 1983; 28:39–45.22. Tötterman TH, Karlsson FA, Bengtsson M, Mendel-Hartvig I. Induction of circulating activated suppressor-like T cells by methimazole therapy for Graves' disease. N Engl J Med. 1987; 316:15–22.23. Wenzel KW, Lente JR. Similar effects of thionamide drugs and perchlorate on thyroid-stimulating immunoglobulins in Graves' disease: evidence against an immunosuppressive action of thionamide drugs. J Clin Endocrinol Metab. 1984; 58:62–9.24. Laurberg P. Remission of Graves' disease during antithyroid drug therapy. Time to reconsider the mechanism? Eur J Endocrinol. 2006; 155:783–6.25. Volpé R. The immunomodulatory effects of anti-thyroid drugs are mediated via actions on thyroid cells, affecting thyrocyte-immunocyte signalling: a review. Curr Pharm Des. 2001; 7:451–60.26. Kourime M, McGowan S, Al Towati M, Ahmed SF, Stewart G, Williamson S, et al. Long-term outcome of thyrotoxicosis in childhood and adolescence in the west of Scotland: the case for long-term antithyroid treatment and the importance of initial counselling. Arch Dis Child. 2018; 103:637–42.27. Kaguelidou F, Carel JC, Léger J. Graves' disease in childhood: advances in management with antithyroid drug therapy. Horm Res. 2009; 71:310–7.28. Gu Y, Liang X, Liu M, Wu D, Li W, Cao B, et al. Clinical features and predictors of remission in children under the age of 7 years with Graves' disease. Pediatr Investig. 2020; 4:198–203.29. Gastaldi R, Poggi E, Mussa A, Weber G, Vigone MC, Salerno M, et al. Graves disease in children: thyroid-stimulating hormone receptor antibodies as remission markers. J Pediatr. 2014; 164:1189–94. e1.30. Jevalikar G, Solis J, Zacharin M. Long-term outcomes of pediatric Graves' disease. J Pediatr Endocrinol Metab. 2014; 27:1131–6.31. El Fassi D, Banga JP, Gilbert JA, Padoa C, Hegedüs L, Nielsen CH. Treatment of Graves' disease with rituximab specifically reduces the production of thyroid stimulating autoantibodies. Clin Immunol. 2009; 130:252–8.32. Diana T, Holthoff HP, Fassbender J, Wüster C, Kanitz M, Kahaly GJ, et al. A novel long-term Graves' disease animal model confirmed by functional thyrotropin receptor antibodies. Eur Thyroid J. 2020; 9(Suppl 1):51–8.33. Pearce SHS, Dayan C, Wraith DC, Barrell K, Olive N, Jansson L, et al. Antigen-specific immunotherapy with thyrotropin receptor peptides in Graves' hyperthyroidism: a phase I study. Thyroid. 2019; 29:1003–11.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The treatment of Graves' disease in children and adolescents
- Commentary on “Remission in pediatric Graves’ disease treated with antithyroid drug and the risk factors associated with relapse”
- Remission Predictors of Graves' Disease in Children
- Clinical Study of Graves' Disease in Children: Remission and Relapse
- Medical Treatment of Graves' Disease