Ann Pediatr Endocrinol Metab.
2022 Dec;27(4):265-272. 10.6065/apem.2244166.083.
Clinical studies of dapagliflozin in pediatric patients: a rapid review
- Affiliations
-
- 1Kentucky Children's Hospital, University of Kentucky Healthcare, Lexington, KY, USA
- 2College of Pharmacy Natural and Health Sciences, Manchester University, Fort Wayne, IN, USA
- KMID: 2537238
- DOI: http://doi.org/10.6065/apem.2244166.083
Abstract
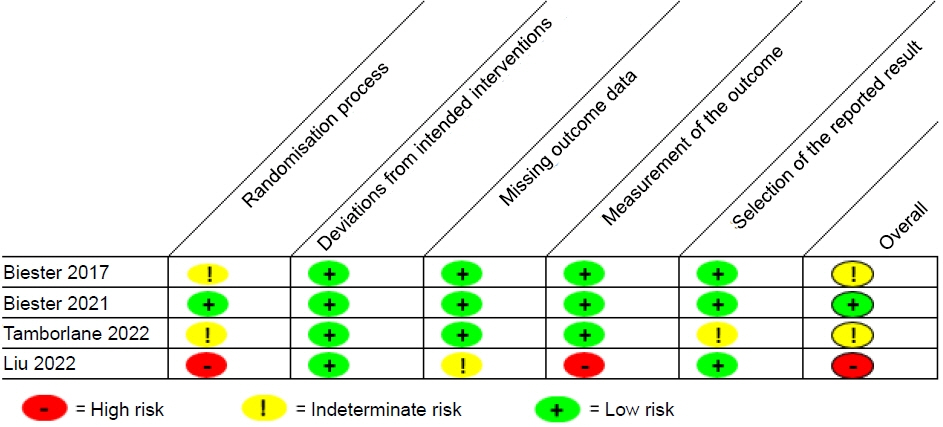
- The oral sodium-glucose cotransporter 2 inhibitor, dapagliflozin, is used to treat kidney disease, heart failure, and diabetes in adults, but has not been well studied in pediatrics, and does not have a recognized place in therapy in current practice guidelines. The purpose of this review is to summarize studies that have investigated the efficacy of dapagliflozin in pediatric patients. A systematic review was performed to identify clinical studies of oral dapagliflozin in children 0 to 17 years. Studies were identified through searches of Scopus, Web of Science, PubMed, Google Scholar, Embase, clinical trial registries, research registries, and key journals through August 2022. The Cochrane scoring system was used to assess the methodological quality of the included randomized trials. Five studies were reviewed and included in this analysis. Dapagliflozin at a dose of 5 to 10 mg was utilized in adolescents and young adults with heart failure, chronic kidney disease with proteinuria, type 1 diabetes, or type 2 diabetes. Studies evaluating dapagliflozin in type 1 diabetes evaluated single doses while the other studies monitored long-term use. Dapagliflozin was overall considered to be safe and effective in the studies included in this review, but further studies in larger populations and over extended periods of time are necessary.
Keyword
Figure
Cited by 1 articles
-
Management of Pediatric Heart Failure
Anne I. Dipchand
Korean Circ J. 2024;54(12):794-810. doi: 10.4070/kcj.2024.0320.
Reference
-
References
1. Farxiga (dapagliflozin) [prescribing information]. Wilmington (DE): AstraZeneca Pharmaceuticals LP; 2021.2. Leoncini G, Russo E, Bussalino E, Barnini C, Viazzi F, Pontremoli R. SGLT2is and renal protection: from biological mechanisms to real-world clinical benefits. Int J Mol Sci. 2021; 22:4441.3. American Diabetes Association. Children and adolescents: standards of medical care in diabetes – 2021. Diabetes Care. 2021; 44(Suppl 1):S180–99.4. Ahmed H, VanderPluym C. Medical management of pediatric heart failure. Cardiovasc Diagn Ther. 2021; 11:323–35.5. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013; 3:1–150.6. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.7. Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inform Libr J. 2009; 26:91–108.8. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898.9. Liu J, Cui J, Fang X, Chen J, Yan W, Shen Q, et al. Efficacy and safety of dapagliflozin in children with inherited proteinuric kidney disease: a pilot study. Kidney Int Rep. 2022; 7:638–41.10. Newland DM, Law YM, Albers EL, Friedland-Little JM, Ahmed H, Kemna MS, et al. Early clinical experience with dapagliflozin in children with heart failure. Pediatr Cardiol. 2022 Aug 10 https://doi.org/10.1007/s00246-022-02983-0. [Epub].11. Biester T, Aschemeier B, Fath M, Frey M, Scheerer M, Kordonouri O, et al. Effects of dapagliflozin on insulin requirement, glucose excretion, and β-hydroxybutyrate levels are not related to baseline HbA1c in youth with type 1 diabetes. Diabetes Obes Metab. 2017; 19:1635–39.12. Biester T, Muller I, von dem Berge T, Atlas E, Nimri R, Phillip M, et al. Add-on therapy with dapagliflozin under full closed loop control improves time in range in adolescents and young adults with type 1 diabetes: the DAPADream study. Diabetes Obes Metab. 2021; 23:599–608.13. Tamborlane W, Laffel L, Shehadeh N, Isganaitis E, Van Name M, Ratnayake J, et al. Efficacy and safety of dapagliflozin in children and young adults with type 2 diabetes: a prospective, multicentre, randomised, parallel group, phase 3 study. Lancet Diabetes Endocrinol. 2022; 10:341–50.14. Roman R, Pereyra M, Ramirez C. Adolescent with type 1 diabetes on insulin and dapagliflozin a SGLT2 inhibitor developed an euglycemic diabetic ketosis. Horm Res Paediatr. 2016; 86(Suppl 2):43.15. Kordonouri O, Biester T, Fath M, Gottwald I, Datz N, Danne T. Add-on treatment with dapagliflozin, a sodium-glucose co-transporter (SGLT) 2 inhibitor, in type 1 diabetes (T1D) – a case report. Pediatr Diabetes. 2015; 16(Suppl 21):124.16. Shamchuk A, Doulla M, Jetha M. Possible association between diabetic ketoacidosis and use of sodium-glucose co-transporter 2 inhibitor in a 17-year-old youth with type 2 diabetes. CMAJ. 2021; 193:E13858–8.17. Newland D, Hong B, Albers E, Friedland-Little J, Kemma M, Hong B, et al. Safety of dapagliflozin in children with heart failure. J Heart Lung Transplant. 2021; 40(4 Suppl):682.18. Tirucherai G, LaCreta F, Ismat F, Tang W, Boulton W. Pharmacokinetics and pharmacodynamics of dapagliflozin in children and adolescents with type 2 diabetes. Diabetes Obes Metab. 2016; 18:678–84.19. Jo H, Pilla Reddy V, Parkinson J, Boulton DW, Tang W. Model-Informed pediatric dose selection for dapagliflozin by incorporat ing developmental changes. CPT Pharmacometrics Syst Pharmacol. 2021; 10:108–18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fournier Gangrene in a Patient With Type 2 Diabetes Mellitus Treated With Dapagliflozin: A Case Report
- Dapagliflozin's Effects on Glycemia and Cardiovascular Risk Factors and Incidence of Adverse Events in Patients with Type 2 Diabetes
- Discrepancies in Dapagliflozin Response in Terms of Glycemic Control and Body Weight Reduction
- Common ABCB1 SNP, C3435T could affect systemic exposure of dapagliflozin in healthy subject
- Comparisons of Efficacy between Dapagliflozin and Sitagliptin in Combination with Metformin in Type 2 Diabetes Mellitus Patients