Ann Surg Treat Res.
2022 Dec;103(6):323-330. 10.4174/astr.2022.103.6.323.
Comprehensive clinical characterization of patients with BRCA1: c.5017_5019del germline variant
- Affiliations
-
- 1Department of Surgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 2Department of Laboratory and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Division of Breast Surgery, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4Breast Cancer Center, Samsung Medical Center, Seoul, Korea
- 5Division of Breast Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Institute of Convergence Medicine Research, Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea
- 7Division of Breast and Endocrine Surgery, Department of Surgery, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 8Department of Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 9Department of Surgery, Soonchunhyang University Bucheon Hospital, Bucheon, Korea
- 10Department of Surgery, Jeonbuk National University Hospital, Jeonbuk National University Medical School, Jeonju, Korea
- 11Department of Surgery, Breast Care Center, Daerim St. Mary’s Hospital, Seoul, Korea
- KMID: 2536908
- DOI: http://doi.org/10.4174/astr.2022.103.6.323
Abstract
- Purpose
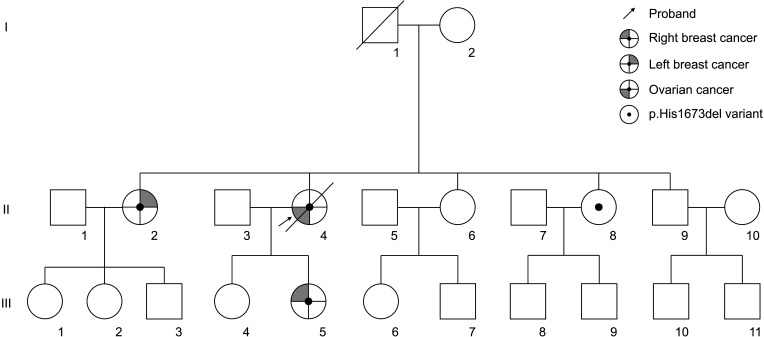
We provide evidence for the reclassification of the BRCA1:c.5017_5019del variant by presenting the clinicopathological characteristics, clinical outcomes, and family history of breast or ovarian cancer in 17 patients with this variant.
Methods
This study included breast or ovarian cancer patients tested for BRCA1/2 genes between January 2008 and June 2020 at 10 medical centers in Korea. We retrospectively reviewed 17 probands from 15 families who had the BRCA1:c.5017_5019del variant according to the electronic medical records.
Results
We present 10 breast cancer patients and 7 ovarian cancer patients from 15 families identified as having BRCA1:c.5017_5019del and a total of 19 cases of breast cancer and 14 cases of ovarian cancer in these families. The ratio of breast-to-ovarian cancer was 1.3:1. Breast cancer patients with this variant showed a rich family history of breast or ovarian cancer, 8 patients (80.0%). The mean age at diagnosis was 45.4 years and 6 patients (60.0%) were categorized into hormone-receptor–negative breast cancer. Also, the ovarian cancer patients with this variant showed strong family histories of breast and/or ovarian cancer in 4 patients (57.1%).
Conclusion
We presented clinical evidence for the reclassification of BRCA1:c.5017_5019del as a likely pathogenic variant (LPV). Reclassification as LPV could result in the prophylactic treatment and medical surveillance of probands, family testing recommendations, and appropriate genetic counseling of their families.
Keyword
Figure
Reference
-
1. Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. 2002; 8:571–576. PMID: 12470990.2. Varol U, Kucukzeybek Y, Alacacioglu A, Somali I, Altun Z, Aktas S, et al. BRCA genes: BRCA 1 and BRCA 2. J BUON. 2018; 23:862–866. PMID: 30358186.3. Rebbeck TR, Friebel TM, Friedman E, Hamann U, Huo D, Kwong A, et al. Mutational spectrum in a worldwide study of 29,700 families with BRCA1 or BRCA2 mutations. Hum Mutat. 2018; 39:593–620. PMID: 29446198.4. Samadder NJ, Riegert-Johnson D, Boardman L, Rhodes D, Wick M, Okuno S, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021; 7:230–237. PMID: 33126242.5. Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021; 19:77–102. PMID: 33406487.6. Warner E, Plewes DB, Hill KA, Causer PA, Zubovits JT, Jong RA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004; 292:1317–1325. PMID: 15367553.7. Brekelmans CT, Seynaeve C, Bartels CC, Tilanus-Linthorst MM, Meijers-Heijboer EJ, Crepin CM, et al. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol. 2001; 19:924–930. PMID: 11181654.8. Caputo SM, Golmard L, Léone M, Damiola F, Guillaud-Bataille M, Revillion F, et al. Classification of 101 BRCA1 and BRCA2 variants of uncertain significance by cosegregation study: a powerful approach. Am J Hum Genet. 2021; 108:1907–1923. PMID: 34597585.9. Kurian AW. BRCA1 and BRCA2 mutations across race and ethnicity: distribution and clinical implications. Curr Opin Obstet Gynecol. 2010; 22:72–78. PMID: 19841585.10. Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009; 115:2222–2233. PMID: 19241424.11. Murray ML, Cerrato F, Bennett RL, Jarvik GP. Follow-up of carriers of BRCA1 and BRCA2 variants of unknown significance: variant reclassification and surgical decisions. Genet Med. 2011; 13:998–1005. PMID: 21811163.12. So MK, Jeong TD, Lim W, Moon BI, Paik NS, Kim SC, et al. Reinterpretation of BRCA1 and BRCA2 variants of uncertain significance in patients with hereditary breast/ovarian cancer using the ACMG/AMP 2015 guidelines. Breast Cancer. 2019; 26:510–519. PMID: 30725392.13. Augusto BM, Lake P, Scherr CL, Couch FJ, Lindor NM, Vadaparampil ST. From the laboratory to the clinic: sharing BRCA VUS reclassification tools with practicing genetics professionals. J Community Genet. 2018; 9:209–215. PMID: 29124491.14. Zuntini R, Cortesi L, Calistri D, Pippucci T, Martelli PL, Casadio R, et al. BRCA1 p.His1673del is a pathogenic mutation associated with a predominant ovarian cancer phenotype. Oncotarget. 2017; 8:22640–22648. PMID: 28186987.15. Lee EH, Park B, Kim NS, Seo HJ, Ko KL, Min JW, et al. The Korean guideline for breast cancer screening. J Korean Med Assoc. 2015; 58:408–419.16. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID: 25741868.17. Rivera-Muñoz EA, Milko LV, Harrison SM, Azzariti DR, Kurtz CL, Lee K, et al. ClinGen Variant Curation Expert Panel experiences and standardized processes for disease and gene-level specification of the ACMG/AMP guidelines for sequence variant interpretation. Hum Mutat. 2018; 39:1614–1622. PMID: 30311389.18. Harrison SM, Biesecker LG, Rehm HL. Overview of specifications to the ACMG/AMP variant interpretation guidelines. Curr Protoc Hum Genet. 2019; 103:e93. PMID: 31479589.19. Maxwell KN, Hart SN, Vijai J, Schrader KA, Slavin TP, Thomas T, et al. Evaluation of ACMG-guideline-based var iant classification of cancer susceptibility and non-cancer-associated genes in families affected by breast cancer. Am J Hum Genet. 2016; 98:801–817. PMID: 27153395.20. Krontiras H, Farmer M, Whatley J. Breast cancer genetics and indications for prophylactic mastectomy. Surg Clin North Am. 2018; 98:677–685. PMID: 30005767.21. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017; 130:e110–e126. PMID: 28832484.22. Friedlander M, Moore KN, Colombo N, Scambia G, Kim BG, Oaknin A, et al. Patient-centred outcomes and effect of disease progression on health status in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation receiving maintenance olaparib or placebo (SOLO1): a randomised, phase 3 trial. Lancet Oncol. 2021; 22:632–642. PMID: 33862001.23. Tutt AN, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021; 384:2394–2405. PMID: 34081848.24. Eggington JM, Bowles KR, Moyes K, Manley S, Esterling L, Sizemore S, et al. A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes. Clin Genet. 2014; 86:229–237. PMID: 24304220.25. Park KS, Cho EY, Nam SJ, Ki CS, Kim JW. Comparative analysis of BRCA1 and BRCA2 variants of uncertain significance in patients with breast cancer: a multifactorial probability-based model versus ACMG standards and guidelines for interpreting sequence variants. Genet Med. 2016; 18:1250–1257. PMID: 27124784.26. Kim JH, Park S, Park HS, Park JS, Lee ST, Kim SW, et al. Analysis of BRCA1/2 variants of unknown significance in the prospective Korean Hereditary Breast Cancer study. Sci Rep. 2021; 11:8485. PMID: 33875706.27. Abou Tayoun AN, Pesaran T, DiStefano MT, Oza A, Rehm HL, Biesecker LG, et al. Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Hum Mutat. 2018; 39:1517–1524. PMID: 30192042.28. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017; 317:2402–2416. PMID: 28632866.29. Ha HI, Ryu JS, Shim H, Kong SY, Lim MC. Reclassification of BRCA1 and BRCA2 variants found in ovarian epithelial, fallopian tube, and primary peritoneal cancers. J Gynecol Oncol. 2020; 31:e83. PMID: 33078592.30. Rebbeck TR, Mitra N, Wan F, Sinilnikova OM, Healey S, McGuffog L, et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA. 2015; 313:1347–1361. PMID: 25849179.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study for Germline Mutation of BRCA1 in Early Onset Breast Cancer Patients
- Novel Germline Mutations of BRCA1 and BRCA2 in Korean Familial Breast Cancer Patients
- Prevalence of germline BRCA mutations among women with carcinoma of the peritoneum or fallopian tube
- Frequency of BRCA1 and BRCA2 Germline Mutations Detected by Protein Truncation Test and Cumulative Risks of Breast and Ovarian Cancer among Mutation Carriers in Japanese Breast Cancer Families
- Colorectal cancer with a germline BRCA1 variant inherited paternally: a case report