J Korean Med Sci.
2022 Dec;37(48):e335. 10.3346/jkms.2022.37.e335.
Outcomes of On-Label Reduced-Dose Edoxaban in Patients With Atrial Fibrillation: The LEDIOS Registry
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Heart Vascular and Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Division of Cardiology, Hallym University Sacred Heart Hospital, Anyang, Korea
- 4Division of Cardiology, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2536731
- DOI: http://doi.org/10.3346/jkms.2022.37.e335
Abstract
- Background
Non-vitamin K antagonist oral anticoagulants (NOACs) are effective in preventing thromboembolisms and reduce the risk of bleeding compared with warfarin. There are few reports on the outcomes of on-label reduced-dose NOACs. The aim of this study was to assess the safety and efficacy of on-label reduced-dose edoxaban in patients with atrial fibrillation (AF).
Methods
This study is a multi-center, prospective, non-interventional study to evaluate the safety and efficacy of on-label reduced-dose edoxaban in patients with AF. We evaluated outcomes of major bleeding, stroke or systemic embolism, all-cause death, and composite clinical outcomes.
Results
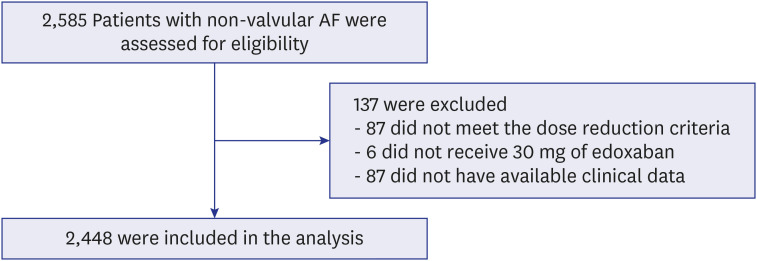
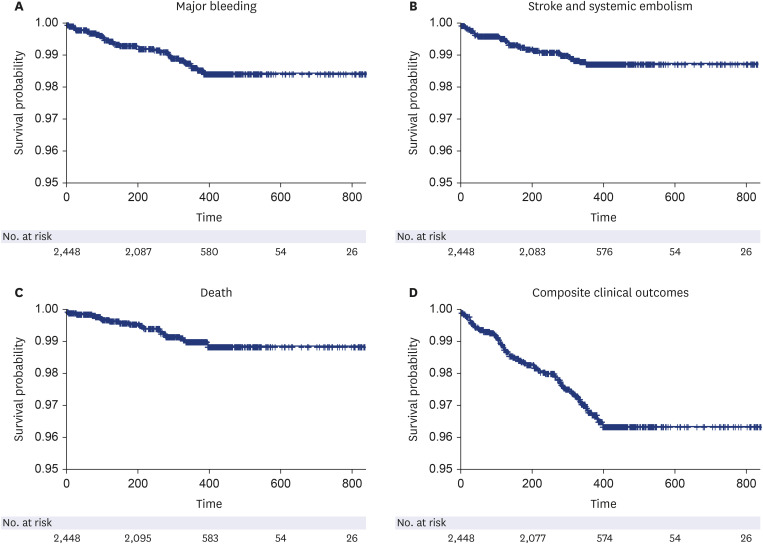
A total of 2,448 patients (mean age 75.0 ± 8.3 years, 801 [32.7%] males) was included in the present study. The mean CHA 2 DS 2 -VASc score was 3.7 ± 1.5. Major bleeding events occurred at a rate of 1.34%/yr. The event rate of strokes and systemic embolisms was 1.13%/ yr. The overall net clinical outcomes occurred at a rate of 3.19%/yr. There were no significant differences according to the number of dose reduction criteria, renal dysfunction, or body weight. Higher HAS-BLED score and higher combination of CHA 2 DS 2 -VASc and HAS-BLED score was associated with an increased risk of composite clinical outcomes compared to the lower score groups.
Conclusions
This study was the largest prospective real-world study to investigate the safety and efficacy of on-label low-dose edoxaban in an Asian population. Reduced-dose edoxaban can be used safely in patients with severe renal dysfunction or extremely low body weight. Our observation suggests that physicians should consider bleeding risk even in a low-dose regimen.
Keyword
Figure
Cited by 1 articles
-
Optimal Dose of Edoxaban for Very Elderly Atrial Fibrillation Patients at High Risk of Bleeding: The LEDIOS Registry
Ju Youn Kim, Juwon Kim, Seung-Jung Park, Kyoung-Min Park, Sang-Jin Han, Dae Kyeong Kim, Yae Min Park, Sung Ho Lee, Jong Sung Park, Young Keun On,
Korean Circ J. 2024;54(7):398-406. doi: 10.4070/kcj.2024.0084.
Reference
-
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021; 42(5):373–498. PMID: 32860505.2. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation. 2019; 140(2):e125–e151. PMID: 30686041.3. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361(12):1139–1151. PMID: 19717844.4. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013; 369(22):2093–2104. PMID: 24251359.5. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365(11):981–992. PMID: 21870978.6. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365(10):883–891. PMID: 21830957.7. Ruff CT, Giugliano RP, Braunwald E, Morrow DA, Murphy SA, Kuder JF, et al. Association between edoxaban dose, concentration, anti-factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015; 385(9984):2288–2295. PMID: 25769361.8. Wang KL, Lopes RD, Patel MR, Büller HR, Tan DS, Chiang CE, et al. Efficacy and safety of reduced-dose non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: a meta-analysis of randomized controlled trials. Eur Heart J. 2019; 40(19):1492–1500. PMID: 30590440.9. Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005; 3(4):692–694. PMID: 15842354.10. Chao TF, Chen SA, Ruff CT, Hamershock RA, Mercuri MF, Antman EM, et al. Clinical outcomes, edoxaban concentration, and anti-factor Xa activity of Asian patients with atrial fibrillation compared with non-Asians in the ENGAGE AF-TIMI 48 trial. Eur Heart J. 2019; 40(19):1518–1527. PMID: 30590425.11. Yamashita T, Koretsune Y, Yang Y, Chen SA, Chung N, Shimada YJ, et al. Edoxaban vs. warfarin in East Asian patients with atrial fibrillation - an ENGAGE AF-TIMI 48 subanalysis. Circ J. 2016; 80(4):860–869. PMID: 26888149.12. Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015; 180:246–254. PMID: 25463377.13. Lee SR, Choi EK, Park SH, Jung JH, Han KD, Oh S, et al. Off-label underdosed apixaban use in Asian patients with non-valvular atrial fibrillation. Eur Heart J Cardiovasc Pharmacother. 2021; 7(5):415–423. PMID: 33471125.14. Lee KN, Choi JI, Boo KY, Kim DY, Kim YG, Oh SK, et al. Effectiveness and safety of off-label dosing of non-vitamin K antagonist anticoagulant for atrial fibrillation in Asian patients. Sci Rep. 2020; 10(1):1801. PMID: 32019993.15. Steffel J, Ruff CT, Yin O, Braunwald E, Park JG, Murphy SA, et al. Randomized, double-blind comparison of half-dose versus full-dose edoxaban in 14,014 patients with atrial fibrillation. J Am Coll Cardiol. 2021; 77(9):1197–1207. PMID: 33663737.16. Morrone D, Kroep S, Ricci F, Renda G, Patti G, Kirchhof P, et al. Mortality prediction of the CHA2DS2-VASc score, the HAS-BLED score, and their combination in anticoagulated patients with atrial fibrillation. J Clin Med. 2020; 9(12):E3987.17. Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GY. Edoxaban in Asian Patients with atrial fibrillation: effectiveness and safety. J Am Coll Cardiol. 2018; 72(8):838–853. PMID: 30115222.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal Dose of Edoxaban for Very Elderly Atrial Fibrillation Patients at High Risk of Bleeding: The LEDIOS Registry
- Application of New Oral Anticoagulants: Prevention of Stroke in Patients with Nonvalvular Atrial Fibrillation
- Low-dose non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation
- Label Adherence for Non-Vitamin K Antagonist Oral Anticoagulants in a Prospective Cohort of Asian Patients with Atrial Fibrillation
- New Oral Anticoagulants: General Features and Review of Pivotal Clinical Trials