COVID-19 Vaccination in Korea: Past, Present, and the Way Forward
- Affiliations
-
- 1Division of Infectious diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 2Vaccine Innovation Center-KU Medicine (VIC-K), Seoul, Korea
- KMID: 2536518
- DOI: http://doi.org/10.3346/jkms.2022.37.e351
Abstract
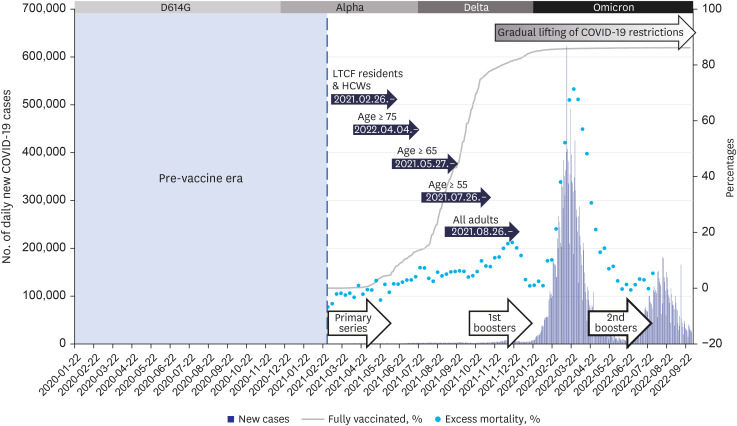
- Since its first emergence in late 2019, severe acute respiratory syndrome coronavirus-2 has claimed more than 6.5 million lives worldwide and continues to infect hundreds of thousands of people daily. To combat this once-in-a-century disaster, several vaccines have been developed at unprecedented speeds. Novel vaccine platforms (messenger ribonucleic acid vaccines and adenoviral vector vaccines) have played a major role in the current pandemic. In Korea, six vaccines, including a domestically developed recombinant vaccine, have been approved. As in other countries, vaccines have been proven to be safe and highly effective in Korea. However, rare serious adverse events and breakthrough infections have undermined public trust in the vaccines, even while the benefits of vaccination far outweigh the risks. The rise of the omicron variant and the subsequent increase in excess mortality demonstrated that while vaccines are a key component of the pandemic response, it alone can fail without non-pharmaceutical interventions like masking and social distancing. The pandemic of coronavirus disease has revealed both the strengths and weaknesses of our healthcare system and pandemic preparedness. When the next pandemic arrives, improved risk communication and vaccine development should be prioritized. To enable timely vaccine development, it is essential to make strategic and sufficient investments in vaccine research and development.
Keyword
Figure
Cited by 4 articles
-
Effects of Omicron Infection and Changes in Serum Antibody Response to Wild-Type, Delta, and Omicron After a Booster Dose With BNT163b2 Vaccine in Korean Healthcare Workers
Sung Hee Lim, Han Jo Kim, Se Hyung Kim, Seong Hyeok Choi, Bora Kim, Ji Youn Kim, Young Sok Ji, Tark Kim, Eun Ju Choo, Jung Chan Jung, Ji Eun Moon, Chan Kyu Kim, Seong Kyu Park, Jina Yun
J Korean Med Sci. 2023;38(13):e103. doi: 10.3346/jkms.2023.38.e103.Eight-Month Follow-up After the Third Dose of BNT162b2 Vaccine in Healthcare Workers: The Question of a Fourth Dose
Sung Hee Lim, Seong Hyeok Choi, Ji Youn Kim, Bora Kim, Han Jo Kim, Se Hyung Kim, Chan Kyu Kim, Seong Kyu Park, Jina Yun
J Korean Med Sci. 2023;38(18):e139. doi: 10.3346/jkms.2023.38.e139.Effective Vaccination and Education Strategies for Emerging Infectious Diseases Such as COVID-19
Seong-Heon Wie, Jaehun Jung, Woo Joo Kim
J Korean Med Sci. 2023;38(44):e371. doi: 10.3346/jkms.2023.38.e371.Cardiovascular Safety of COVID-19 Vaccination in Patients With Cancer: A Self-Controlled Case Series Study in Korea
Ji Hwa Ryu, Ahhyung Choi, Jieun Woo, Hyesung Lee, Jinkwon Kim, Joonsang Yoo, Ju-Young Shin
J Korean Med Sci. 2024;39(24):e190. doi: 10.3346/jkms.2024.39.e190.
Reference
-
1. Kim JY, Choe PG, Oh Y, Oh KJ, Kim J, Park SJ, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020; 35(5):e61. PMID: 32030925.2. WHO. Coronavirus disease (COVID-19) pandemic. Updated September 29, 2022. Accessed September 29, 2022. https://www.who.int/europe/emergencies/situations/covid-19 .3. Cumulative confirmed COVID-19 deaths. Updated September 28, 2022. Accessed September 29, 2022. https://ourworldindata.org/coronavirus#explore-the-global-situation .4. COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention. Coronavirus disease-19: the first 7,755 cases in the Republic of Korea. Osong Public Health Res Perspect. 2020; 11(2):85–90. PMID: 32257774.5. Kang SJ, Jung SI. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020; 52(2):154–164. PMID: 32537961.6. Kim M, Yoo JR, Heo ST, Lee HR, Oh H. Clinical characteristics and risk factors for severe disease of coronavirus disease 2019 in a low case fatality rate region in Korea. Infect Chemother. 2021; 53(4):718–729. PMID: 34951535.7. Agca M, Tuncay E, Yıldırım E, Yıldız R, Sevim T, Ernam D, et al. Is obesity a potential risk factor for poor prognosis of COVID-19? Infect Chemother. 2021; 53(2):319–331. PMID: 34216125.8. Hoang T, Tran Thi Anh T. Comparison of comorbidities in relation to critical conditions among coronavirus disease 2019 patients: a network meta-analysis. Infect Chemother. 2021; 53(1):13–28. PMID: 34409779.9. Kim Y, Kim SW, Chang HH, Kwon KT, Bae S, Hwang S. Significance and associated factors of long-term sequelae in patients after acute COVID-19 infection in Korea. Infect Chemother. 2021; 53(3):463–476. PMID: 34405592.10. Kim Y, Kim SE, Kim T, Yun KW, Lee SH, Lee E, et al. Preliminary guidelines for the clinical evaluation and management of long COVID. Infect Chemother. 2022; 54(3):566–597. PMID: 36196612.11. KDCA. COVID-19 vaccination safety report. Updated September 25, 2022. Accessed September 29, 2022. https://ncv.kdca.go.kr/board.es?mid=a11707010000&bid=0032# .12. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018; 17(4):261–279. PMID: 29326426.13. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.14. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384(5):403–416. PMID: 33378609.15. Rosa SS, Prazeres DM, Azevedo AM, Marques MP. mRNA vaccines manufacturing: challenges and bottlenecks. Vaccine. 2021; 39(16):2190–2200. PMID: 33771389.16. Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, et al. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. BMJ. 2022; 378:e069445. PMID: 35830976.17. Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022; 19(2):75–77. PMID: 34887571.18. CDC. Update on myocarditis following mRNA COVID-19 vaccination. Updated June 7, 2022. Accessed September 15, 2022. https://www.fda.gov/media/159007/download .19. EMA. New vaccine for prevention of Ebola virus disease recommended for approval in the European Union. Updated May 29, 2020. Accessed September 15, 2022. https://www.ema.europa.eu/en/documents/press-release/new-vaccine-prevention-ebola-virus-disease-recommended-approval-european-union_en.pdf .20. WHO. Ebola virus disease - Democratic Republic of Congo External Situation Report 98. Updated June 23, 2020. Accessed September 15, 2022. https://apps.who.int/iris/bitstream/handle/10665/332654/SITREP_EVD_DRC_20200623-eng.pdf .21. Yang TC, Millar JB, Grinshtein N, Bassett J, Finn J, Bramson JL. T-cell immunity generated by recombinant adenovirus vaccines. Expert Rev Vaccines. 2007; 6(3):347–356. PMID: 17542750.22. Fausther-Bovendo H, Kobinger GP. Pre-existing immunity against Ad vectors: humoral, cellular, and innate response, what’s important? Hum Vaccin Immunother. 2014; 10(10):2875–2884. PMID: 25483662.23. Liu X, Shaw RH, Stuart AS, Greenland M, Aley PK, Andrews NJ, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021; 398(10303):856–869. PMID: 34370971.24. Vivaldi G, Jolliffe DA, Holt H, Tydeman F, Talaei M, Davies GA, et al. Risk factors for SARS-CoV-2 infection after primary vaccination with ChAdOx1 nCoV-19 or BNT162b2 and after booster vaccination with BNT162b2 or mRNA-1273: a population-based cohort study (COVIDENCE UK). Lancet Reg Health Eur. 2022; 22:100501. PMID: 36168404.25. Menni C, May A, Polidori L, Louca P, Wolf J, Capdevila J, et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study. Lancet Infect Dis. 2022; 22(7):1002–1010. PMID: 35405090.26. Pavord S, Scully M, Hunt BJ, Lester W, Bagot C, Craven B, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021; 385(18):1680–1689. PMID: 34379914.27. Acosta-Coley I, Cervantes-Ceballos L, Tejeda-Benítez L, Sierra-Márquez L, Cabarcas-Montalvo M, García-Espiñeira M, et al. Vaccines platforms and COVID-19: what you need to know. Trop Dis Travel Med Vaccines. 2022; 8(1):20. PMID: 35965345.28. Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022; 386(6):531–543. PMID: 34910859.29. Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021; 385(13):1172–1183. PMID: 34192426.30. Song J, Choi W, Kwon K, Jung D, Choi H, Cheong H. Phase 3 Study of Safety and Immunogenicity of GBP510 Vaccine Adjuvanted With AS03 in Adults (Abstract Number: 828), International Congress on Infectious Diseases (ISID 2022). Brookline, MA, USA: International Society for Infectious Diseases;2022.31. Lim S, Lee Y, Kim DW, Park WS, Yoon JH, Lee JY. Anti-SARS-CoV-2 neutralizing antibody responses after two doses of ChAdOx1 nCoV-19 vaccine (AZD1222) in healthcare workers. Infect Chemother. 2022; 54(1):140–152. PMID: 35384425.32. Kang YM, Minn D, Lim J, Lee K-D, Jo DH, Choe K-W, et al. Comparison of antibody response elicited by ChAdOx1 and BNT162b2 COVID-19 vaccine. J Korean Med Sci. 2021; 36(46):e311. PMID: 34845875.33. Lee HJ, Jung J, Lee JH, Lee DG, Kim YB, Oh EJ. Comparison of six serological immunoassays for the detection of SARS-CoV-2 neutralizing antibody levels in the vaccinated population. Viruses. 2022; 14(5):946. PMID: 35632688.34. Hyun H, Choi MJ, Heo JY, Seo YB, Nham E, Yoon JG, et al. Immunogenicity and reactogenicity of Ad26.COV2.S in Korean adults: a prospective cohort study. J Korean Med Sci. 2022; 37(27):e210. PMID: 35818701.35. Bae S, Ko JH, Choi JY, Park WJ, Lim SY, Ahn JY, et al. Heterologous ChAdOx1 and Bnt162b2 vaccination induces strong neutralizing antibody responses against SARS-CoV-2 including delta variant with tolerable reactogenicity. Clin Microbiol Infect. 2022; 28(10):1390.e1–1390.e7.36. Hillus D, Schwarz T, Tober-Lau P, Vanshylla K, Hastor H, Thibeault C, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021; 9(11):1255–1265. PMID: 34391547.37. KDCA. Analysis of the preventive effect of the fourth coronavirus disease vaccine dose in immunocompromised individuals and residents of elderly care hospitals and facilities. Updated July 14, 2022. Accessed September 17, 2022. https://kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&list_no=720134&act=view .38. KDCA. A fourth COVID-19 vaccination in 60+. Updated April 13, 2022. Accessed October 1, 2022. http://ncov.kdca.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6567&contSeq=6567&board_id=312&gubun=BDJ .39. Kang CK, Shin HM, Choe PG, Park J, Hong J, Seo JS, et al. Broad humoral and cellular immunity elicited by one-dose mRNA vaccination 18 months after SARS-CoV-2 infection. BMC Med. 2022; 20(1):181. PMID: 35508998.40. Choi MJ, Choi JY, Hyun H, Nham E, Seong H, Yoon JG, et al. Cross-neutralization of omicron subvariants after heterologous NVX-CoV2373 boosters: comparison between prior SARS-CoV-2-infected and infection-naive individuals. J Infect. 2022; DOI: 10.1016/j.jinf.2022.09.018.41. Noh JY, Cheong HJ, Kim WJ, Choi JY, Lee HW, Kim SS, et al. Robust neutralizing antibody responses after single-dose BNT162b2 vaccination at long intervals from prior SARS-CoV-2 infection and ceiling effect with repeated vaccination. J Infect. 2022; 85(5):573–607.42. Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021; 27(6):981–984. PMID: 33795870.43. Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021; 384(14):1372–1374. PMID: 33691060.44. Yi S, Choe YJ, Lim DS, Lee HR, Kim J, Kim YY, et al. Impact of national Covid-19 vaccination campaign, South Korea. Vaccine. 2022; 40(26):3670–3675. PMID: 35570077.45. Kim C, Kang G, Kang SG, Lee H. COVID-19 outbreak response at a nursing hospital in South Korea in the post-vaccination era, including an estimation of the effectiveness of the first shot of the Oxford-AstraZeneca COVID-19 vaccine (ChAdOx1-S). Osong Public Health Res Perspect. 2022; 13(2):114–122. PMID: 35538683.46. KDCA. A Korea COVID-19 vaccine effectiveness (K-COVE) study published on clinical infectious diseases. Updated 2022. Accessed September 17, 2022. https://www.korea.kr/news/pressReleaseView.do?newsId=156504574 .47. KDCA. COV-EPI evaluation study in LTCF. Updated May 12, 2022. Accessed September 17, 2022. https://kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&list_no=719529&act=view .48. Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION network, 10 States, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(4):139–145. PMID: 35085224.49. Zhu Y, Liu S, Zhang D. Effectiveness of COVID-19 vaccine booster shot compared with non-booster: a meta-analysis. Vaccines (Basel). 2022; 10(9):1396. PMID: 36146474.50. Kim SR, Kang HJ, Jeong HR, Jang SY, Lee JE, Kim DE, et al. Relative effectiveness of COVID-19 vaccination in healthcare workers: 3-dose versus 2-dose vaccination. J Korean Med Sci. 2022; 37(35):e267. PMID: 36065651.51. Grewal R, Kitchen SA, Nguyen L, Buchan SA, Wilson SE, Costa AP, et al. Effectiveness of a fourth dose of covid-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: test negative design study. BMJ. 2022; 378:e071502. PMID: 35793826.52. Nordström P, Ballin M, Nordström A. Effectiveness of a fourth dose of mRNA COVID-19 vaccine against all-cause mortality in long-term care facility residents and in the oldest old: a nationwide, retrospective cohort study in Sweden. Lancet Reg Health Eur. 2022; 21:100466. PMID: 35855494.53. June Choe Y, Yi S, Hwang I, Kim J, Park YJ, Cho E, et al. Safety and effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents. Vaccine. 2022; 40(5):691–694. PMID: 35012777.54. Hachmann NP, Miller J, Collier AY, Ventura JD, Yu J, Rowe M, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022; 387(1):86–88. PMID: 35731894.55. Tseng HF, Ackerson BK, Bruxvoort KJ, Sy LS, Tubert JE, Lee GS, et al. Effectiveness of mRNA-1273 against infection and COVID-19 hospitalization with SARS-CoV-2 Omicron subvariants: BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. medRxiv. October 1, 2022. DOI: 10.1101/2022.09.30.22280573.56. Møller Kirsebom FC, Andrews N, Stowe J, Groves N, Chand M, Ramsay M, et al. Effectiveness of the COVID-19 vaccines against hospitalisation with omicron sub-lineages BA.4 and BA.5 in England. Lancet Reg Health Eur. 2022; 23:100537. PMID: 36381141.57. Surie D, Bonnell L, Adams K, Gaglani M, Ginde AA, Douin DJ, et al. Effectiveness of monovalent mRNA vaccines against COVID-19-associated hospitalization among immunocompetent adults during BA.1/BA.2 and BA.4/BA.5 predominant periods of SARS-CoV-2 omicron variant in the United States - IVY network, 18 States, December 26, 2021–August 31, 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(42):1327–1334. PMID: 36264830.58. Tartof SY, Slezak JM, Puzniak L, Hong V, Frankland TB, Ackerson BK, et al. BNT162b2 vaccine effectiveness against SARS-CoV-2 omicron BA.4 and BA.5. Lancet Infect Dis. 2022; 22(12):1663–1665.59. Collie S, Nayager J, Bamford L, Bekker LG, Zylstra M, Gray G. Effectiveness and durability of the BNT162b2 vaccine against omicron sublineages in South Africa. N Engl J Med. 2022; 387(14):1332–1333. PMID: 36103455.60. Pfizer-BioNTech. Pfizer and BioNTech announce omicron-adapted COVID-19 vaccine candidates demonstrate high immune response against omicron. Updated June 25, 2022. Accessed November 16, 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-omicron-adapted-covid-19 .61. Moderna. Moderna’s BA.4/BA.5 targeting bivalent booster, mRNA-1273.222, meets primary endpoint of superiority against omicron variants compared to booster dose of mRNA-1273 in phase 2/3 clinical trial. Updated November 14, 2022. Accessed November 16, 2022. https://investors.modernatx.com/news/news-details/2022/Modernas-BA.4BA.5-Targeting-Bivalent-Booster-mRNA-1273.222-Meets-Primary-Endpoint-of-Superiority-Against-Omicron-Variants-Compared-to-Booster-Dose-of-mRNA-1273-in-Phase-23-Clinical-Trial/default.aspx# .62. Pfizer-BioNTech. Pfizer/BioNTech COVID-19 omicron-modified bivalent vaccine. Updated September 1, 2022. Accessed November 16, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/07-covid-swanson-508.pdf .63. Scheaffer SM, Lee D, Whitener B, Ying B, Wu K, Jani H, et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice. Nat Med. Forthcoming 2022. DOI: 10.1056/NEJMc2210093.64. Collier AY, Miller J, Hachmann NP, McMahan K, Liu J, Bondzie EA, et al. Immunogenicity of the BA.5 bivalent mRNA vaccine boosters. bioRxiv. October 25, 2022. DOI: 10.1101/2022.10.24.513619.65. Link-Gelles R, Ciesla AA, Fleming-Dutra KE, Smith ZR, Britton A, Wigegand RE, et al. Effectiveness of bivalent mRNA vaccines in preventing symptomatic SARS-CoV-2 infection — increasing community access to testing program, United States, September–November 2022. Updated 2022. Accessed November 23, 2022. https://www.cdc.gov/mmwr/volumes/71/wr/mm7148e1.htm .66. Hyun H, Song JY, Seong H, Yoon JG, Noh JY, Cheong HJ, et al. Polyarthralgia and myalgia syndrome after ChAdOx1 nCOV-19 vaccination. J Korean Med Sci. 2021; 36(34):e245. PMID: 34463066.67. Lee JH, Oh SM, Lee E, Bang JH, Park SW. Treatment for immune thrombocytopenia in coronavirus disease 2019 (COVID-19) infection after COVID-19 vaccination: a case report. Infect Chemother. 2022; 54(3):559–562. PMID: 35132832.68. Park JW, Yu SN, Chang SH, Ahn YH, Jeon MH. Multisystem inflammatory syndrome in an adult after COVID-19 vaccination: a case report and literature review. J Korean Med Sci. 2021; 36(45):e312. PMID: 34811978.69. Kang HS, Kim JE, Yoo JR, Oh H, Kim M, Kim YR, et al. Aseptic meningitis following second dose of an mRNA coronavirus disease 2019 vaccine in a healthy male: case report and literature review. Infect Chemother. 2022; 54(1):189–194. PMID: 35132836.70. Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021; 384(23):2187–2201. PMID: 33882225.71. Hwang I, Park K, Kim TE, Kwon Y, Lee YK. COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021. Osong Public Health Res Perspect. 2021; 12(6):396–402. PMID: 34965689.72. Maltezou HC, Anastassopoulou C, Hatziantoniou S, Poland GA, Tsakris A. Anaphylaxis rates associated with COVID-19 vaccines are comparable to those of other vaccines. Vaccine. 2022; 40(2):183–186. PMID: 34863620.73. Song JE, Oh GB, Park HK, Lee SS, Kwak YG. Survey of adverse events after the first dose of the ChAdOx1 nCoV-19 vaccine: a single-center experience in Korea. Infect Chemother. 2021; 53(3):557–561. PMID: 34405593.74. Jeon M, Kim J, Oh CE, Lee JY. Adverse events following immunization associated with coronavirus disease 2019 vaccination reported in the mobile vaccine adverse events reporting system. J Korean Med Sci. 2021; 36(17):e114. PMID: 33942578.75. Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021; 36(17):e115. PMID: 33942579.76. Kim SH, Wi YM, Yun SY, Ryu JS, Shin JM, Lee EH, et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021; 36(14):e107. PMID: 33847085.77. Lee YW, Lim SY, Lee JH, Lim JS, Kim M, Kwon S, et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Korean Med Sci. 2021; 36(21):e153. PMID: 34060261.78. Kim S, Hwang I, Ko M, Kwon Y, Lee YK. Safety monitoring of COVID-19 vaccination among adolescents aged 12 to 17 years old in the Republic of Korea. Osong Public Health Res Perspect. 2022; 13(3):230–237. PMID: 35820672.79. Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021; 385(3):239–250. PMID: 34043894.80. Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D, et al. COVID-19 vaccine safety in adolescents aged 12–17 years - United States, December 14, 2020–July 16, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(31):1053–1058. PMID: 34351881.81. Lee CW, Sa S, Hong M, Kim J, Shim SR, Han HW. Adverse events and safety profile of the COVID-19 vaccines in adolescents: safety monitoring for adverse events using real-world data. Vaccines (Basel). 2022; 10(5):744. PMID: 35632500.82. Principi N, Esposito S. Vaccine-preventable diseases, vaccines and Guillain-Barre’ syndrome. Vaccine. 2019; 37(37):5544–5550. PMID: 29880241.83. Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet. 2013; 381(9876):1461–1468. PMID: 23498095.84. Coronavirus vaccine - summary of Yellow Card reporting. Updated September 1, 2022. Accessed September 25, 2022. https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting .85. Reported side effects following COVID-19 vaccination in Canada. Updated September 2, 2022. Accessed September 25, 2022. https://health-infobase.canada.ca/covid-19/vaccine-safety/ .86. Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022; 327(4):331–340. PMID: 35076665.87. KDCA. National Vaccine Injury Compensation Program. Updated 2022. Accessed November 16, 2022. https://ncv.kdca.go.kr/menu.es?mid=a12602000000 .88. Kim J, Yoo Y, Bang E, Kim S. A comparison of the implementation of national COVID-19 vaccine injury compensation system. Public Health Wkly Rep. 2022; 15(39):2653–2665.89. KDCA. Strengthening goverment support for vaccine injury compensation. Updated July 19, 2022. Accessed November 16, 2022. https://ncov.kdca.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6763&contSeq=6763&board_id=312&gubun=BDJ .90. Noh EB, Nam HK, Lee H. Which group should be vaccinated first?: a systematic review. Infect Chemother. 2021; 53(2):261–270. PMID: 34216120.91. Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021; 384(22):2124–2130. PMID: 33835768.92. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021; 384(22):2092–2101. PMID: 33835769.93. Shi HJ, Nham E, Kim B, Joo EJ, Cheong HS, Hong SH, et al. Clinical characteristics and risk factors for mortality in critical coronavirus disease 2019 patients 50 years of age or younger during the delta wave: comparison with patients > 50 years in Korea. J Korean Med Sci. 2022; 37(22):e175. PMID: 35668685.94. Liu L, Iketani S, Guo Y, Chan JF, Wang M, Liu L, et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature. 2022; 602(7898):676–681. PMID: 35016198.95. Cumulative confirmed COVID-19 cases per million people. Updated September 29, 2022. Accessed September 30, 2022. https://ourworldindata.org/coronavirus#explore-the-global-situation .96. Excess mortality: Deaths from all causes compared to average over previous years. Updated July 31, 2022. Accessed October 1, 2022. https://ourworldindata.org/grapher/excess-mortality-p-scores-average-baseline?country=~KOR .97. KDCA. Current status of COVID-19 outbreak and vaccination in Korea. Updated September 29, 2022. Accessed September 30, 2022. http://ncov.kdca.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6897&contSeq=6897&board_id=312&gubun=BDJ .98. KDCA. Booster plans against COVID-19, Winter 2022–2023: press release. Updated September 21, 2022. Accessed September 30, 2022. http://ncov.kdca.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=6850&contSeq=6850&board_id=312&gubun=BDJ .99. Laidlaw BJ, Ellebedy AH. The germinal centre B cell response to SARS-CoV-2. Nat Rev Immunol. 2022; 22(1):7–18. PMID: 34873279.100. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022; 23(2):186–193. PMID: 35105982.101. Hägg S, Religa D. COVID vaccination in older adults. Nat Microbiol. 2022; 7(8):1106–1107. PMID: 35836001.102. Duong MC, Nguyen HT, Duong BT. Who influences the public intention to get a COVID-19 vaccine and what are the public references and concerns? A population survey in Vietnam. Infect Chemother. 2021; 53(4):753–766. PMID: 34979606.103. Lamptey E. Should breakthrough SARS-CoV-2 infection affect our confidence in the COVID-19 vaccines? Infect Chemother. 2021; 53(4):676–685. PMID: 34979603.104. Choi WS, Cheong HJ. COVID-19 vaccination for people with comorbidities. Infect Chemother. 2021; 53(1):155–158. PMID: 34409789.105. MFDS. Domestic COVID-19 vaccine candidates in clinical trials. Updated November 1, 2022. Accessed November 18, 2022. https://www.mfds.go.kr/docviewer/skin/doc.html?fn=20220805094804221.png&rs=/docviewer/result/co19vaccine01/1/1/202211 .106. Novavax and SK bioscience Expand Manufacturing Agreement. Updated December 23, 2021. Accessed November 18, 2022. https://ir.novavax.com/2021-12-23-Novavax-and-SK-bioscience-Expand-Manufacturing-Agreement .107. Moderna and Samsung Biologics Announce Agreement for Fill-Finish Manufacturing of Moderna’s COVID-19 Vaccine. Updated August 13, 2021. Accessed November 18, 2022. https://samsungbiologics.com/kr/media/company-news-view?boardSeq=1207&schBoardCtgryCcd=1&schString=&schBoardYear=&boardDtm=1621699200000&page=2 .108. IVI. IVI to operate training courses for WHO global biomanufacturing training hub in Republic of Korea. Updated March 29, 2022. Accessed November 18, 2022. https://www.ivi.int/ivi-to-operate-training-courses-for-who-global-biomanufacturing-training-hub-in-republic-of-korea/ .109. Peck KR. Collaborative response to COVID-19 pandemic, and development of treatment guidelines. Infect Chemother. 2021; 53(1):151–154. PMID: 34409788.110. Ministry of Food and Drug Safety. COVID-19 vaccines. Updated November 1, 2022. Accessed November 14, 2022. https://www.mfds.go.kr/docviewer/skin/doc.html?fn=20221101084746428.hwp&rs=/docviewer/result/co19vaccine01/5/1/202211 .111. Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021; 385(25):2348–2360. PMID: 34587382.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Aphthous Stomatitis in a Healthy Adult Following COVID-19 Vaccination: Clinical Reasoning
- The Role of COVID-19 Vaccination for Patients With Atherosclerotic Cardiovascular Disease in the Upcoming Endemic Era
- Factors Influencing the COVID-19 Vaccination Intentions in Nurses: Korea, February 2021
- Vestibular Neuritis after COVID-19 Vaccination
- Early-Onset Myasthenia Gravis Following COVID-19 Vaccination