J Pathol Transl Med.
2022 Nov;56(6):342-353. 10.4132/jptm.2022.08.31.
A clinicopathologic and immunohistochemical study of primary and secondary breast angiosarcoma
- Affiliations
-
- 1Department of Pathology, Wayne State University School of Medicine/Detroit Medical Center, Detroit, MI, USA
- 2Biostatistics and Bioinformatics Core, Karmanos Cancer Institute, Department of Oncology, Wayne State University School of Medicine, Detroit, MI, USA
- KMID: 2535854
- DOI: http://doi.org/10.4132/jptm.2022.08.31
Abstract
- Background
We aimed to study the clinicopathologic and immunohistochemical (IHC) (CD117, c-Myc, and p53) characteristics, and overall survival of primary and secondary breast angiosarcoma (BAS).
Methods
This was a retrospective study of BAS cases diagnosed between 1997 and 2020 at our institution. Hematoxylin and eosin-stained slides were reviewed for tumor morphology, margin status, and lymph node metastasis. CD117, p53, D2-40, CD31, and c-Myc IHC stains were performed on 11 viable tissue blocks. Additional clinical information was obtained from the electronic medical records.
Results
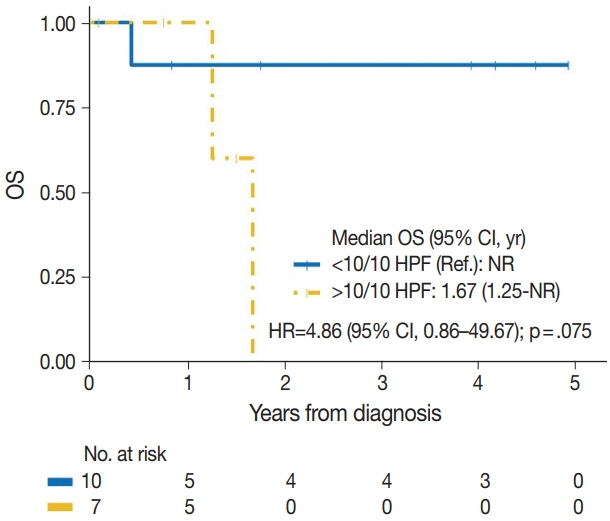
Seventeen patients with BAS were identified. Of these, five (29%) were primary and 12 (71%) were secondary BAS, respectively. The median age at diagnosis for primary BAS was 36 years. The median age at diagnosis for secondary BAS was 67 years. The median time to secondary BAS development following radiotherapy was 6.5 years (range, 2 to 12 years). There was no significant difference between primary and secondary BAS in several histopathologic parameters examined, including histologic grade, necrosis, mitotic count, lymph node metastasis, and positive tumor margins. There was also no difference in CD117, p53, D2-40, CD31, and c-Myc expression by IHC between primary and secondary BAS. During a median followup of 21 months, primary BAS had two (40%) reported deaths and secondary BAS had three (25%) reported deaths. However, this difference in survival between both groups was not statistically significant (hazard ratio, 0.51; 95% confidence interval, 0.09 to 3.28; p = .450).
Conclusions
BAS is a rare and aggressive disease. No histologic, IHC (CD117, c-Myc, and p53), or survival differences were identified between primary and secondary BAS in this study.
Keyword
Figure
Reference
-
References
1. Abdou Y, Elkhanany A, Attwood K, Ji W, Takabe K, Opyrchal M. Primary and secondary breast angiosarcoma: single center report and a meta-analysis. Breast Cancer Res Treat. 2019; 178:523–33.
Article2. Brar R, West R, Witten D, Raman B, Jacobs C, Ganjoo K. Breast angiosarcoma: case series and expression of vascular endothelial growth factor. Case Rep Oncol. 2009; 2:242–50.
Article3. Kunkiel M, Maczkiewicz M, Jagiello-Gruszfeld A, Nowecki Z. Primary angiosarcoma of the breast-series of 11 consecutive cases: a single-centre experience. Curr Oncol. 2018; 25:e50–3.4. Yin M, Wang W, Drabick JJ, Harold HA. Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: a comparative study. BMC Cancer. 2017; 17:295.
Article5. Torres KE, Ravi V, Kin K, et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann Surg Oncol. 2013; 20:1267–74.
Article6. Arora TK, Terracina KP, Soong J, Idowu MO, Takabe K. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014; 3:28–34.7. Moore A, Hendon A, Hester M, Samayoa L. Secondary angiosarcoma of the breast: can imaging findings aid in the diagnosis? Breast J. 2008; 14:293–8.
Article8. Kaur RP, Vasudeva K, Kumar R, Munshi A. Role of p53 gene in breast cancer: focus on mutation spectrum and therapeutic strategies. Curr Pharm Des. 2018; 24:3566–75.
Article9. Croce CM, Thierfelder W, Erikson J, et al. Transcriptional activation of an unrearranged and untranslocated c-myc oncogene by translocation of a C lambda locus in Burkitt. Proc Natl Acad Sci U S A. 1983; 80:6922–6.
Article10. Mastronikolis N, Ragos V, Kyrodimos E, et al. Mechanisms of Cmyc oncogenic activity in head and neck squamous cell carcinoma. J BUON. 2019; 24:2242–4.11. Georgakopoulos G, Tsiambas E, Korkolopoulos P, et al. c-MYC and h-TERT co-expression in colon adenocarcinoma: a tissue microarray digitized image analysis. J BUON. 2013; 18:124–30.12. La Rosa S, Bernasconi B, Vanoli A, et al. c-MYC amplification and c-myc protein expression in pancreatic acinar cell carcinomas. New insights into the molecular signature of these rare cancers. Virchows Arch. 2018; 473:435–41.
Article13. Tsiambas E, Stamatelopoulos A, Baltayiannis N, et al. Evaluation of combined telomerase and c-myc expression in non-small cell lung carcinomas using tissue microarrays and computerized image analysis. J BUON. 2005; 10:533–9.14. Miettinen M, Sarlomo-Rikala M, Lasota J. KIT expression in angiosarcomas and fetal endothelial cells: lack of mutations of exon 11 and exon 17 of C-kit. Mod Pathol. 2000; 13:536–41.
Article15. Billings SD, McKenney JK, Folpe AL, Hardacre MC, Weiss SW. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004; 28:781–8.
Article16. de Bree E, van Coevorden F, Peterse JL, Russell NS, Rutgers EJ. Bilateral angiosarcoma of the breast after conservative treatment of bilateral invasive carcinoma: genetic predisposition? Eur J Surg Oncol. 2002; 28:392–5.
Article17. Komdeur R, Hoekstra HJ, Molenaar WM, et al. Clinicopathologic assessment of postradiation sarcomas: KIT as a potential treatment target. Clin Cancer Res. 2003; 9:2926–32.18. Lae M, Lebel A, Hamel-Viard F, et al. Can c-myc amplification reliably discriminate postradiation from primary angiosarcoma of the breast? Cancer Radiother. 2015; 19:168–74.
Article19. Hung J, Hiniker SM, Lucas DR, et al. Sporadic versus radiation-associated angiosarcoma: a comparative clinicopathologic and molecular analysis of 48 cases. Sarcoma. 2013; 2013:798403.
Article20. Italiano A, Chen CL, Thomas R, et al. Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer. 2012; 118:5878–87.
Article21. Cordon-Cardo C, Reuter VE. Alterations of tumor suppressor genes in bladder cancer. Semin Diagn Pathol. 1997; 14:123–32.22. Mallofre C, Castillo M, Morente V, Sole M. Immunohistochemical expression of CK20, p53, and Ki-67 as objective markers of urothelial dysplasia. Mod Pathol. 2003; 16:187–91.
Article23. Nwanze J, Siddiqui MT, Stevens KA, Saxe D, Cohen C. MYC immunohistochemistry Predicts MYC Rearrangements by FISH. Front Oncol. 2017; 7:209.
Article24. Donnell RM, Rosen PP, Lieberman PH, et al. Angiosarcoma and other vascular tumors of the breast. Am J Surg Pathol. 1981; 5:629–42.
Article25. Rosen PP, Kimmel M, Ernsberger D. Mammary angiosarcoma: the prognostic significance of tumor differentiation. Cancer. 1988; 62:2145–51.
Article26. Wang XY, Jakowski J, Tawfik OW, Thomas PA, Fan F. Angiosarcoma of the breast: a clinicopathologic analysis of cases from the last 10 years. Ann Diagn Pathol. 2009; 13:147–50.
Article27. Hui A, Henderson M, Speakman D, Skandarajah A. Angiosarcoma of the breast: a difficult surgical challenge. Breast. 2012; 21:584–9.
Article28. Seinen JM, Styring E, Verstappen V, et al. Radiation-associated angiosarcoma after breast cancer: high recurrence rate and poor survival despite surgical treatment with R0 resection. Ann Surg Oncol. 2012; 19:2700–6.
Article29. Jallali N, James S, Searle A, Ghattaura A, Hayes A, Harris P. Surgical management of radiation-induced angiosarcoma after breast conservation therapy. Am J Surg. 2012; 203:156–61.
Article30. Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012; 19:3801–8.
Article31. Brenn T, Fletcher CD. Radiation-associated cutaneous atypical vascular lesions and angiosarcoma: clinicopathologic analysis of 42 cases. Am J Surg Pathol. 2005; 29:983–96.32. Hornick JL, Fletcher CD. Immunohistochemical staining for KIT (CD117) in soft tissue sarcomas is very limited in distribution. Am J Clin Pathol. 2002; 117:188–93.
Article33. Shet T, Malaviya A, Nadkarni M, et al. Primary angiosarcoma of the breast: observations in Asian Indian women. J Surg Oncol. 2006; 94:368–74.
Article34. Requena C, Rubio L, Lavernia J, et al. Immunohistochemical and fluorescence in situ hybridization analysis of MYC in a series of 17 cutaneous angiosarcomas: a single-center study. Am J Dermatopathol. 2018; 40:349–54.
Article35. Vorburger SA, Xing Y, Hunt KK, et al. Angiosarcoma of the breast. Cancer. 2005; 104:2682–8.
Article36. Sher T, Hennessy BT, Valero V, et al. Primary angiosarcomas of the breast. Cancer. 2007; 110:173–8.
Article37. Marchal C, Weber B, de Lafontan B, et al. Nine breast angiosarcomas after conservative treatment for breast carcinoma: a survey from French comprehensive Cancer Centers. Int J Radiat Oncol Biol Phys. 1999; 44:113–9.
Article38. Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX study. J Clin Oncol. 2008; 26:5269–74.
Article