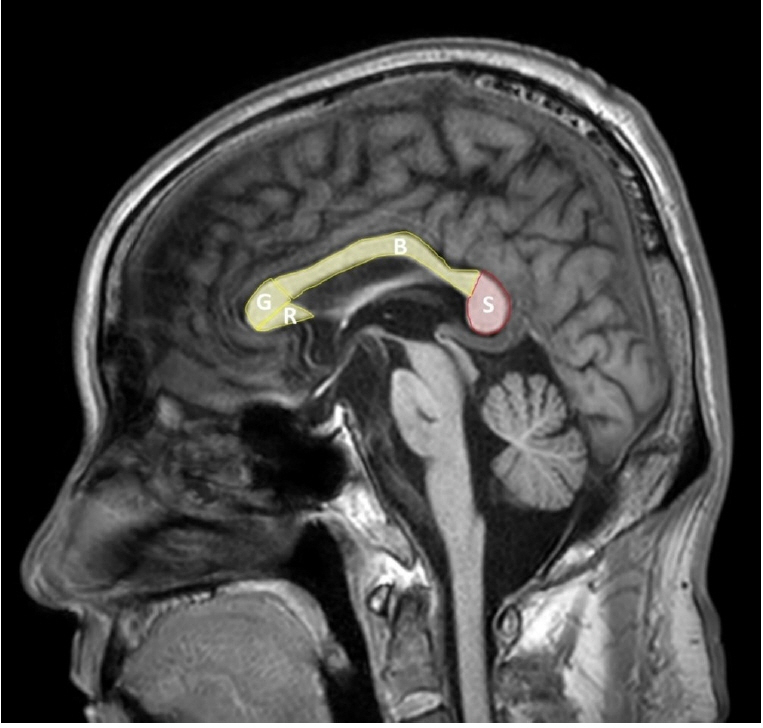
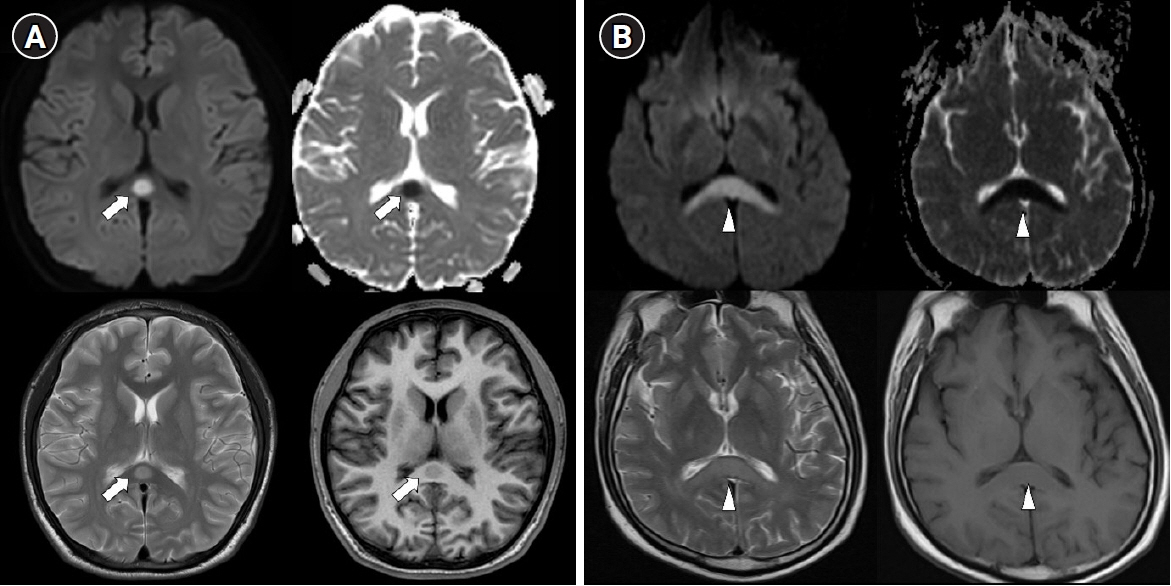
Acute Crit Care.
2022 Aug;37(3):269-275. 10.4266/acc.2022.00864.
Transient splenial lesions of the corpus callosum and infectious diseases
- Affiliations
-
- 1Department of Neurology, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
- KMID: 2535289
- DOI: http://doi.org/10.4266/acc.2022.00864
Abstract
- comTransient splenial lesion of the corpus callosum can be observed in various diseases such as cancer, drug use, metabolic disorders, and cerebrovascular disorders, as well as in patients with infectious diseases. During the coronavirus disease 2019 (COVID-19) pandemic, there were increasing reports of these lesions being detected on brain imaging tests performed in patients with neurological symptoms. On brain magnetic resonance imaging, findings suggestive of cytotoxic edema are observed in the splenium; these are known to disappear with improvement of clinical symptoms. Cytokinopathy caused by infection increases the permeability of the blood–brain barrier and activates the glial cells of the brain to induce cytotoxic edema. Most patients have a good prognosis. The causes, mechanism, diagnosis, treatment and prognosis of transient splenial lesions of the corpus callosum will be summarized in this review.
Figure
Reference
-
1. Knyazeva MG. Splenium of corpus callosum: patterns of interhemispheric interaction in children and adults. Neural Plast. 2013; 2013:639430.2. Raybaud C. The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology. 2010; 52:447–77.
Article3. Takanashi J, Hirasawa K, Tada H. Reversible restricted diffusion of entire corpus callosum. J Neurol Sci. 2006; 247:101–4.
Article4. Starkey J, Kobayashi N, Numaguchi Y, Moritani T. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics. 2017; 37:562–76.
Article5. Garcia-Monco JC, Cortina IE, Ferreira E, Martínez A, Ruiz L, Cabrera A, et al. Reversible splenial lesion syndrome (RESLES): what's in a name? J Neuroimaging. 2011; 21:e1–14.
Article6. Takanashi J, Barkovich AJ, Shiihara T, Tada H, Kawatani M, Tsukahara H, et al. Widening spectrum of a reversible splenial lesion with transiently reduced diffusion. AJNR Am J Neuroradiol. 2006; 27:836–8.7. Bulakbasi N, Kocaoglu M, Tayfun C, Ucoz T. Transient splenial lesion of the corpus callosum in clinically mild influenza-associated encephalitis/encephalopathy. AJNR Am J Neuroradiol. 2006; 27:1983–6.8. Singh P, Gogoi D, Vyas S, Khandelwal N. Transient splenial lesion: further experience with two cases. Indian J Radiol Imaging. 2010; 20:254–7.
Article9. Malhotra HS, Garg RK, Vidhate MR, Sharma PK. Boomerang sign: clinical significance of transient lesion in splenium of corpus callosum. Ann Indian Acad Neurol. 2012; 15:151–7.
Article10. Cho JS, Ha SW, Han YS, Park SE, Hong KM, Han JH, et al. Mild encephalopathy with reversible lesion in the splenium of the corpus callosum and bilateral frontal white matter. J Clin Neurol. 2007; 3:53–6.
Article11. Kakadia B, Ahmed J, Siegal T, Jovin TG, Thon JM. Mild encephalopathy with reversible splenium lesion (MERS) in a patient with COVID-19. J Clin Neurosci. 2020; 79:272–4.
Article12. Kahilogullari G, Comert A, Ozdemir M, Brohi RA, Ozgural O, Esmer AF, et al. Arterial vascularization patterns of the splenium: an anatomical study. Clin Anat. 2013; 26:675–81.
Article13. Mathews MS, Linskey ME, Binder DK, William P. Van Wagenen and the first corpus callosotomies for epilepsy. J Neurosurg. 2008; 108:608–13.14. Lin D, Rheinboldt M. Reversible splenial lesions presenting in conjunction with febrile illness: a case series and literature review. Emerg Radiol. 2017; 24:599–604.
Article15. Talukder NT, Feezel A, Lankford JE. Mild encephalitis/encephalopathy with a reversible splenial lesion associated with systemic Mycoplasma pneumoniae infection in North America: a case report. J Med Case Rep. 2022; 16:74.
Article16. Mawatari M, Kobayashi T, Yamamoto S, Takeshita N, Hayakawa K, Kutsuna S, et al. Mild encephalitis/encephalopathy with a reversible splenial lesion due to Plasmodium falciparum malaria: a case report. Trop Med Health. 2018; 46:37.
Article17. Sathananthasarma P, Weeratunga PN, Chang T. Reversible splenial lesion syndrome associated with dengue fever: a case report. BMC Res Notes. 2018; 11:412.
Article18. Varol F, Ergul N, Sahin EG, Can YY, Ergul U, Guven S, et al. Can plasma exchange therapy be an option for the treatment of SARS-CoV-2 related splenial lesion syndrome: two cases from the pediatric intensive care unit. Transfus Apher Sci. 2022; 103491.
Article19. Arıkan FA, Akdağ G, Çetiner M, Uysal N, Kabay SC. Isolated corpus callosum lesion associated with cytokine storm in COVID-19. Proc (Bayl Univ Med Cent). 2022; 35:337–8.
Article20. DE Oliveira FA, DE Melo TF, Rocha-Filho PA. Transient lesion in the splenium of the corpus callosum associated with COVID-19. Arq Neuropsiquiatr. 2020; 78:738.
Article21. Sawlani V, Scotton S, Nader K, Jen JP, Patel M, Gokani K, et al. COVID-19-related intracranial imaging findings: a large single-centre experience. Clin Radiol. 2021; 76:108–16.
Article22. McLeod NA, Williams JP, Machen B, Lum GB. Normal and abnormal morphology of the corpus callosum. Neurology. 1987; 37:1240–2.
Article23. Park MK, Hwang SH, Jung S, Hong SS, Kwon SB. Lesions in the splenium of the corpus callosum: clinical and radiological implications. Neurol Asia. 2014; 19:79–88.24. Chen WX, Liu HS, Yang SD, Zeng SH, Gao YY, Du ZH, et al. Reversible splenial lesion syndrome in children: retrospective study and summary of case series. Brain Dev. 2016; 38:915–27.
Article25. Baek SH, Shin DI, Lee HS, Lee SH, Kim HY, Shin KS, et al. Reversible splenium lesion of the corpus callosum in hemorrhagic fever with renal failure syndrome. J Korean Med Sci. 2010; 25:1244–6.
Article26. Tada H, Takanashi J, Barkovich AJ, Oba H, Maeda M, Tsukahara H, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. 2004; 63:1854–8.
Article27. Doherty MJ, Jayadev S, Watson NF, Konchada RS, Hallam DK. Clinical implications of splenium magnetic resonance imaging signal changes. Arch Neurol. 2005; 62:433–7.
Article28. Takanashi J, Tada H, Maeda M, Suzuki M, Terada H, Barkovich AJ. Encephalopathy with a reversible splenial lesion is associated with hyponatremia. Brain Dev. 2009; 31:217–20.
Article29. Miyata R, Tanuma N, Hayashi M, Imamura T, Takanashi J, Nagata R, et al. Oxidative stress in patients with clinically mild encephalitis/encephalopathy with a reversible splenial lesion (MERS). Brain Dev. 2012; 34:124–7.
Article30. Motobayashi M, Fukuyama T, Okuno-Yuguchi J, Tsukahara K, Nagaharu S, Hagimoto R, et al. Subclinical neuroaxonal damage in patients with clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Pediatr Neurol. 2017; 74:e3–4.
Article31. Moritani T, Smoker WR, Sato Y, Numaguchi Y, Westesson PL. Diffusion-weighted imaging of acute excitotoxic brain injury. AJNR Am J Neuroradiol. 2005; 26:216–28.32. Conklin J, Frosch MP, Mukerji SS, Rapalino O, Maher MD, Schaefer PW, et al. Susceptibility-weighted imaging reveals cerebral microvascular injury in severe COVID-19. J Neurol Sci. 2021; 421:117308.
Article33. Sparr SA, Bieri PL. Infarction of the splenium of the corpus callosum in the age of COVID-19: a snapshot in time. Stroke. 2020; 51:e223–6.34. Chougar L, Shor N, Weiss N, Galanaud D, Leclercq D, Mathon B, et al. Retrospective observational study of brain MRI findings in patients with acute SARS-CoV-2 infection and neurologic manifestations. Radiology. 2020; 297:E313–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transient Splenial Lesion of the Corpus Callosum in Patients with Infectious Disease
- A Case of Transient Isolated Splenial Lesion of the Corpus Callosum After New Onset Seizure
- Reversible Splenial Lesion in the Corpus Callosum on MRI after Ingestion of a Herbicide Containing Glufosinate Ammonium: A Case Report
- Lithium-Induced Downbeat Nystagmus with Reversible Splenial Lesion
- Recurrent Clinically Mild Encephalitis/Encephalopathy with a Reversible Splenial Lesion (MERS) on Diffusion Weighted Imaging: A Case Report