Brain Tumor Res Treat.
2022 Oct;10(4):255-264. 10.14791/btrt.2022.0035.
Clinical Features and Prognosis of Diffuse Midline Glioma: A Series of 24 Cases
- Affiliations
-
- 1Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2534724
- DOI: http://doi.org/10.14791/btrt.2022.0035
Abstract
- Background
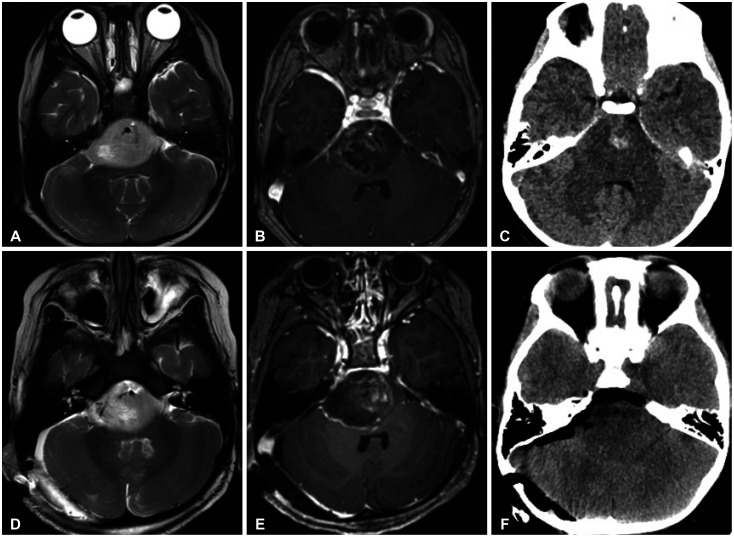
Diffuse midline glioma (DMG) which occurs in midline structures and characterized by harboring K27M mutation in genes encoding the histone 3 protein is classified as World Health Organization (WHO) grade IV regardless of histological findings and has a poor prognosis. Nevertheless, because of its relatively rare incidence compared with other high-grade gliomas, a comprehensive description encompassing clinical features and genomic profiles of DMG is still lacking.
Methods
In this study, we analyzed data of 24 patients who were diagnosed as DMG which was confirmed by surgical specimens in both pediatric and adult patients. We described the clinical outcomes of patients with DMG and their genomic profiles through a retrospective analysis of 24 patients with DMG.
Results
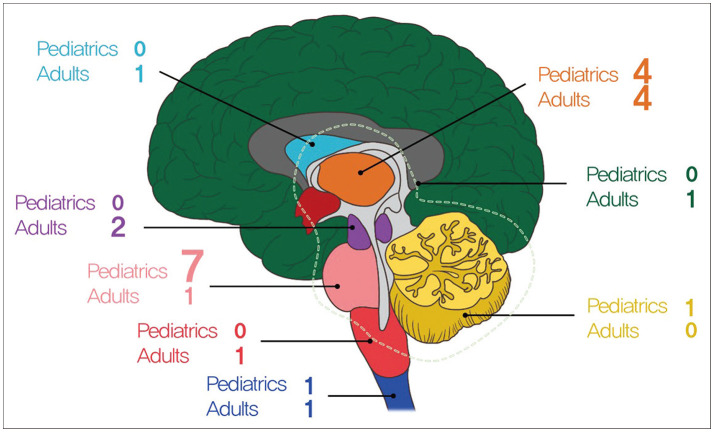
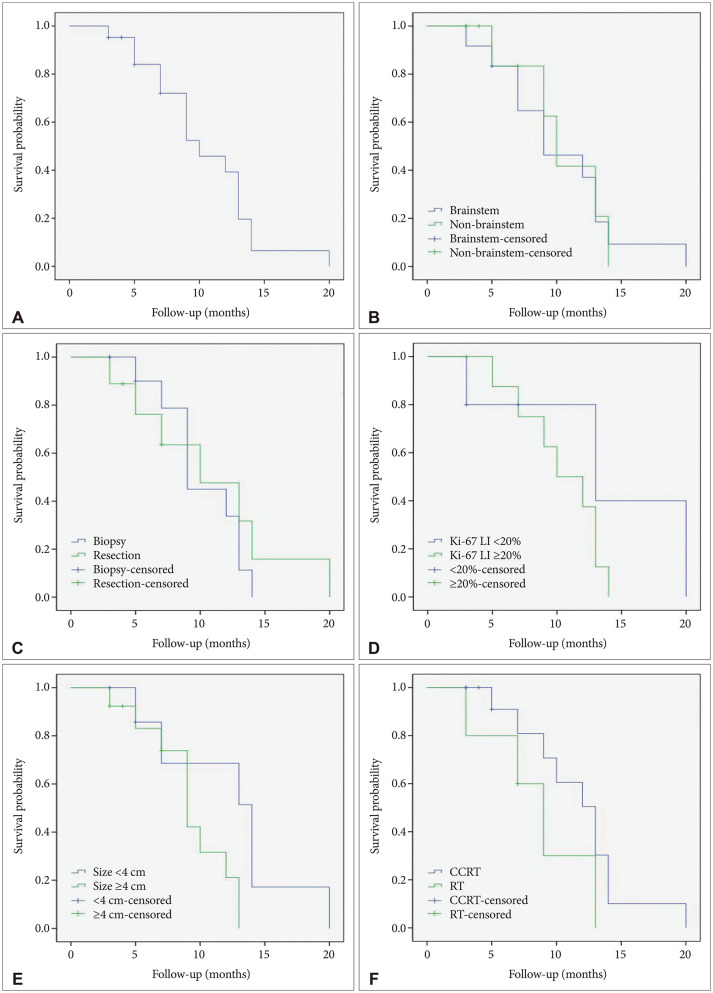
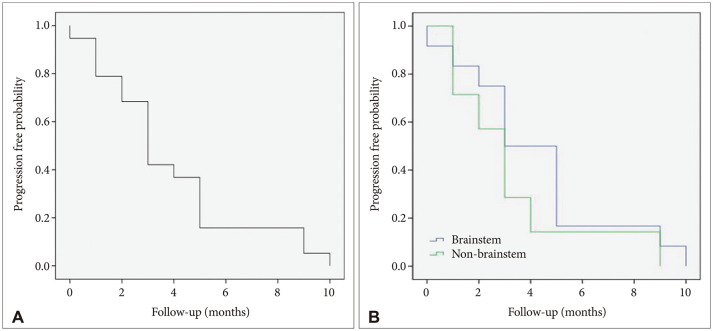
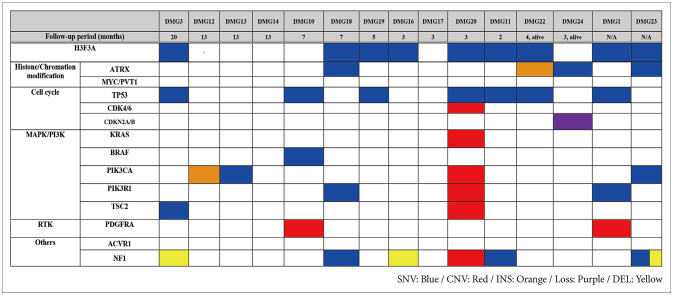
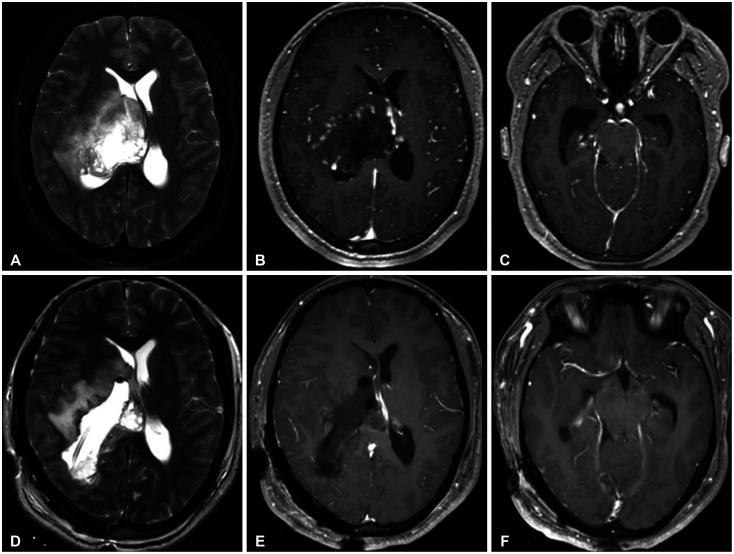
The clinical characteristics of the 24 patients with DMG were analyzed. Ten patients (41%) underwent tumor resection and 14 patients (59%) underwent tumor biopsy. The median overall survival was 10.4 months (95% confidence interval [CI], 8.4 to 12.5) and progression free survival was 3.9 months (95% CI, 2.6 to 5.2). Fifteen patients (62%) were accompanied by hydrocephalus. None of the patient, tumor, or treatment factors had any significant associated with survival. In both immunohistochemistry staining (n=24) and targeted next generation sequencing (n=15), TP53 mutation was the most common genetic mutation (25% and 46%, respectively) found in the patients except alterations in histone 3 protein.
Conclusion
Although surgical treatment of patient with DMG does not affect the overall survival prognosis, it can help improve the patient’s accompanying neurological symptoms in some limited cases. Hydrocephalus is often accompanied with DMG and treatment for hydrocephalus is often also required. Multidisciplinary therapeutic approach is needed.
Keyword
Figure
Reference
-
1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131:803–820. PMID: 27157931.
Article2. Gielen GH, Gessi M, Hammes J, Kramm CM, Waha A, Pietsch T. H3F3A K27M mutation in pediatric CNS tumors: a marker for diffuse high-grade astrocytomas. Am J Clin Pathol. 2013; 139:345–349. PMID: 23429371.3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23:1231–1251. PMID: 34185076.
Article4. Chan KM, Fang D, Gan H, Hashizume R, Yu C, Schroeder M, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013; 27:985–990. PMID: 23603901.
Article5. El-Khouly FE, Veldhuijzen van Zanten SEM, Santa-Maria Lopez V, Hendrikse NH, Kaspers GJL, Loizos G, et al. Diagnostics and treatment of diffuse intrinsic pontine glioma: where do we stand? J Neurooncol. 2019; 145:177–184. PMID: 31522324.
Article6. Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M, et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018; 20:123–131. PMID: 29016894.
Article7. Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016; 26:569–580. PMID: 26517431.
Article8. Schreck KC, Ranjan S, Skorupan N, Bettegowda C, Eberhart CG, Ames HM, et al. Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J Neurooncol. 2019; 143:87–93. PMID: 30864101.
Article9. Wang L, Li Z, Zhang M, Piao Y, Chen L, Liang H, et al. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol. 2018; 78:89–96. PMID: 29727696.
Article10. Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, Uro-Coste E, Amiel-Benouaich A, et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017; 19:1127–1134. PMID: 28201752.
Article11. Schulte JD, Buerki RA, Lapointe S, Molinaro AM, Zhang Y, Villanueva-Meyer JE, et al. Clinical, radiologic, and genetic characteristics of histone H3 K27M-mutant diffuse midline gliomas in adults. Neurooncol Adv. 2020; 2:vdaa142. PMID: 33354667.
Article12. Kleinschmidt-DeMasters BK, Mulcahy Levy JM. H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol. 2018; 37:53–63. PMID: 29393845.
Article13. Sturm D, Pfister SM, Jones DTW. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J Clin Oncol. 2017; 35:2370–2377. PMID: 28640698.
Article14. Aihara K, Mukasa A, Gotoh K, Saito K, Nagae G, Tsuji S, et al. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol. 2014; 16:140–146. PMID: 24285547.
Article15. Bin-Alamer O, Jimenez AE, Azad TD, Bettegowda C, Mukherjee D. H3K27M-altered diffuse midline gliomas among adult patients: a systematic review of clinical features and survival analysis. World Neurosurg. 2022; 165:e251–e264. PMID: 35697228.
Article16. Yoon HI, Wee CW, Kim YZ, Seo Y, Im JH, Dho YS, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for adult diffuse midline glioma: version 2021.1. Brain Tumor Res Treat. 2021; 9:1–8. PMID: 33913265.17. Banan R, Christians A, Bartels S, Lehmann U, Hartmann C. Absence of MGMT promoter methylation in diffuse midline glioma, H3 K27M-mutant. Acta Neuropathol Commun. 2017; 5:98. PMID: 29246238.
Article18. Abe H, Natsumeda M, Kanemaru Y, Watanabe J, Tsukamoto Y, Okada M, et al. MGMT expression contributes to temozolomide resistance in H3K27M-mutant diffuse midline gliomas and MGMT silencing to temozolomide sensitivity in IDH-mutant gliomas. Neurol Med Chir (Tokyo). 2018; 58:290–295. PMID: 29848907.19. Vuong HG, Le HT, Ngo TNM, Fung KM, Battiste JD, McNall-Knapp R, et al. H3K27M-mutant diffuse midline gliomas should be further molecularly stratified: an integrated analysis of 669 patients. J Neurooncol. 2021; 155:225–234. PMID: 34796414.
Article20. Cooney TM, Lubanszky E, Prasad R, Hawkins C, Mueller S. Diffuse midline glioma: review of epigenetics. J Neurooncol. 2020; 150:27–34. PMID: 32804378.
Article21. Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017; 32:520–537.e5. PMID: 28966033.22. Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014; 46:451–456. PMID: 24705254.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffuse Midline Gliomas Harboring the H3 K27M-Mutation in the Bilateral Thalamus and Midbrain: A Case Report and a Review of the Literature
- Clinical and Genetic Features of Brainstem Glioma in Adults: A Report of 50 Cases in a Single Center
- Epigenetic and Metabolic Changes in Diffuse Intrinsic Pontine Glioma
- Hemorrhagic Recurrence in Diffuse Astrocytoma without Malignant Transformation
- CT classification & Prognosis of head injury