Endocrinol Metab.
2022 Oct;37(5):770-780. 10.3803/EnM.2022.1519.
Association among Current Smoking, Alcohol Consumption, Regular Exercise, and Lower Extremity Amputation in Patients with Diabetic Foot: Nationwide Population-Based Study
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Statistics and Actuarial Science, Soongsil University, Seoul, Korea
- KMID: 2534629
- DOI: http://doi.org/10.3803/EnM.2022.1519
Abstract
- Background
The present study investigates whether modifiable behavioral factors of current cigarette smoking, heavy alcohol consumption, and regular exercise are associated with risk of lower extremity amputation (LEA) in diabetic patients.
Methods
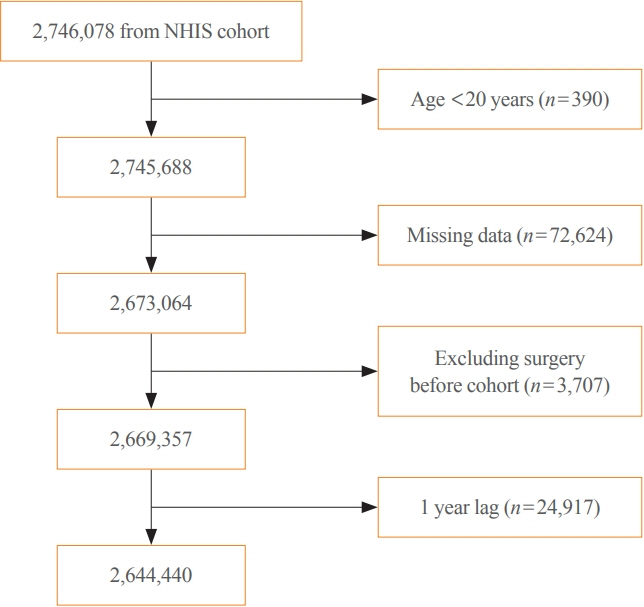
A total of 2,644,440 diabetic patients (aged ≥20 years) was analyzed using the database of the Korean National Health Insurance Service. Cox proportional hazard regression was used to assess adjusted hazard ratios (HRs) for the behavioral factors with risk of LEA under adjustment for potential confounders.
Results
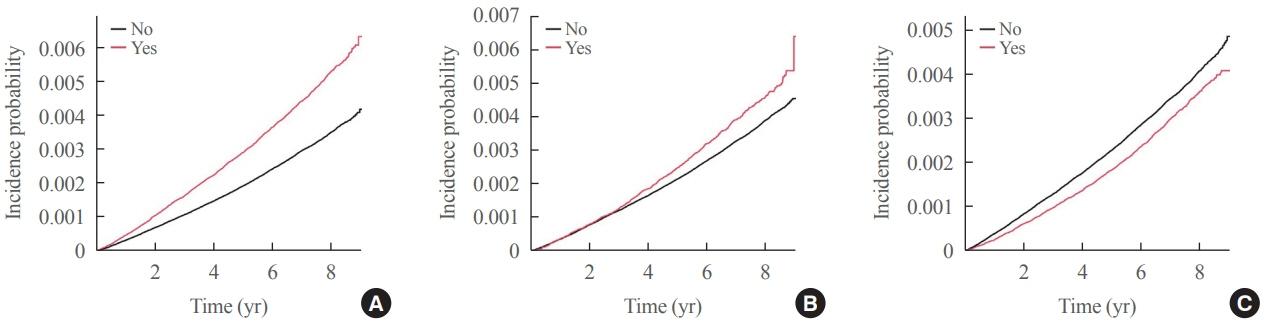
The risk of LEA was significantly increased by current cigarette smoking and heavy alcohol consumption (HR, 1.436; 95% confidence interval [CI], 1.367 to 1.508 and HR, 1.082; 95% CI, 1.011 to 1.158) but significantly decreased with regular exercise (HR, 0.745; 95% CI, 0.706 to 0.786) after adjusting for age, sex, smoking, alcohol consumption, exercise, low income, hypertension, dyslipidemia, body mass index, using insulin or oral antidiabetic drugs, and diabetic duration. A synergistically increased risk of LEA was observed with larger number of risky behaviors.
Conclusion
Modification of behaviors of current smoking, heavy alcohol intake, and exercise prevents LEA and can improve physical, emotional, and social quality of life in diabetic patients.
Figure
Reference
-
1. Apelqvist J. Diagnostics and treatment of the diabetic foot. Endocrine. 2012; 41:384–97.
Article2. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017; 49:106–16.
Article3. Mishra SC, Chhatbar KC, Kashikar A, Mehndiratta A. Diabetic foot. BMJ. 2017; 359:j5064.
Article4. Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Diabetic foot syndrome: evaluating the prevalence and incidence of foot pathology in Mexican Americans and non-Hispanic whites from a diabetes disease management cohort. Diabetes Care. 2003; 26:1435–8.5. Reiber GE. The epidemiology of diabetic foot problems. Diabet Med. 1996; 13 Suppl 1:S6–11.
Article6. Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al. Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes. Health Technol Assess. 2009; 13:1–86.
Article7. Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications. 2000; 14:235–41.
Article8. Kerr M, Rayman G, Jeffcoate WJ. Cost of diabetic foot disease to the National Health Service in England. Diabet Med. 2014; 31:1498–504.
Article9. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017; 376:2367–75.
Article10. Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system: the contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998; 21:855–9.
Article11. Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003; 26:491–4.
Article12. Ikonen TS, Sund R, Venermo M, Winell K. Fewer major amputations among individuals with diabetes in Finland in 1997-2007: a population-based study. Diabetes Care. 2010; 33:2598–603.13. Ragnarson Tennvall G, Apelqvist J. Health-economic consequences of diabetic foot lesions. Clin Infect Dis. 2004; 39 Suppl 2:S132–9.
Article14. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014; 37:651–8.
Article15. Apelqvist J, Ragnarson-Tennvall G, Persson U, Larsson J. Diabetic foot ulcers in a multidisciplinary setting: an economic analysis of primary healing and healing with amputation. J Intern Med. 1994; 235:463–71.
Article16. Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005; 366:1719–24.
Article17. Monteiro-Soares M, Boyko EJ, Ribeiro J, Ribeiro I, DinisRibeiro M. Predictive factors for diabetic foot ulceration: a systematic review. Diabetes Metab Res Rev. 2012; 28:574–600.
Article18. Parisi MC, Moura Neto A, Menezes FH, Gomes MB, Teixeira RM, de Oliveira JE, et al. Baseline characteristics and risk factors for ulcer, amputation and severe neuropathy in diabetic foot at risk: the BRAZUPA study. Diabetol Metab Syndr. 2016; 8:25.
Article19. Papanas N, Maltezos E. Glycated hemoglobin as a risk factor for lower extremity amputations in diabetes: “success is counted sweetest”. Int J Low Extrem Wounds. 2015; 14:106–7.20. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46:e15.
Article21. Yang HK, Han K, Kwon HS, Park YM, Cho JH, Yoon KH, et al. Obesity, metabolic health, and mortality in adults: a nationwide population-based study in Korea. Sci Rep. 2016; 6:30329.
Article22. Bae EH, Lim SY, Jung JH, Oh TR, Choi HS, Kim CS, et al. Chronic kidney disease risk of isolated systolic or diastolic hypertension in young adults: a nationwide sample basedcohort study. J Am Heart Assoc. 2021; 10:e019764.
Article23. Gulliford MC, Mahabir D. Diabetic foot disease and foot care in a Caribbean community. Diabetes Res Clin Pract. 2002; 56:35–40.
Article24. Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes: the independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999; 22:1029–35.
Article25. Riaz M, Miyan Z, Zaidi SI, Alvi SF, Fawwad A, Ahmadani MY, et al. Characteristics of a large cohort of patients with diabetes having at-risk feet and outcomes in patients with foot ulceration referred to a tertiary care diabetes unit. Int Wound J. 2016; 13:594–9.
Article26. Tseng CH. Prevalence and risk factors of diabetic foot problems in Taiwan: a cross-sectional survey of non-type 1 diabetic patients from a nationally representative sample. Diabetes Care. 2003; 26:3351.27. Iversen MM, Tell GS, Riise T, Hanestad BR, Ostbye T, Graue M, et al. History of foot ulcer increases mortality among individuals with diabetes: ten-year follow-up of the Nord-Trondelag Health Study, Norway. Diabetes Care. 2009; 32:2193–9.28. Bus SA, van Deursen RW, Armstrong DG, Lewis JE, Caravaggi CF, Cavanagh PR, et al. Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab Res Rev. 2016; 32 Suppl 1:99–118.
Article29. Ward R, Dunn J, Clavijo L, Shavelle D, Rowe V, Woo K. Outcomes of critical limb ischemia in an urban, safety net hospital population with high WIfI amputation scores. Ann Vasc Surg. 2017; 38:84–9.
Article30. Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993; 233:485–91.
Article31. Lazzarini PA, Hurn SE, Fernando ME, Jen SD, Kuys SS, Kamp MC, et al. Prevalence of foot disease and risk factors in general inpatient populations: a systematic review and meta-analysis. BMJ Open. 2015; 5:e008544.
Article32. Holman N, Young RJ, Jeffcoate WJ. Variation in the recorded incidence of amputation of the lower limb in England. Diabetologia. 2012; 55:1919–25.
Article33. Liu M, Zhang W, Yan Z, Yuan X. Smoking increases the risk of diabetic foot amputation: a meta-analysis. Exp Ther Med. 2018; 15:1680–5.
Article34. Lakier JB. Smoking and cardiovascular disease. Am J Med. 1992; 93(1A):8S–12S.
Article35. Kourembanas S, Marsden PA, McQuillan LP, Faller DV. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991; 88:1054–7.
Article36. Lee FS. Genetic causes of erythrocytosis and the oxygensensing pathway. Blood Rev. 2008; 22:321–32.
Article37. Ben G, Gnudi L, Maran A, Gigante A, Duner E, Iori E, et al. Effects of chronic alcohol intake on carbohydrate and lipid metabolism in subjects with type II (non-insulin-dependent) diabetes. Am J Med. 1991; 90:70–6.
Article38. Pal B, Raveender N, Sudipta P. A study on the impact of smoking and alcoholism as determinant factors in the prognosis and outcome of diabetic foot ulcer disease. Int J Res Med Sci. 2016; 4:1720–4.
Article39. Matos M, Mendes R, Silva AB, Sousa N. Physical activity and exercise on diabetic foot related outcomes: a systematic review. Diabetes Res Clin Pract. 2018; 139:81–90.
Article40. Sartor CD, Hasue RH, Cacciari LP, Butugan MK, Watari R, Passaro AC, et al. Effects of strengthening, stretching and functional training on foot function in patients with diabetic neuropathy: results of a randomized controlled trial. BMC Musculoskelet Disord. 2014; 15:137.
Article41. Tran MM, Haley MN. Does exercise improve healing of diabetic foot ulcers?: a systematic review. J Foot Ankle Res. 2021; 14:19.
Article42. Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014; 14:528.
Article43. Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006; 20:216–23.
Article44. Ahn S, Song R. Effects of Tai Chi exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy. J Altern Complement Med. 2012; 18:1172–8.
Article45. Lemaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008; 88:1385–98.
Article46. Moura Neto A, Zantut-Wittmann DE, Fernandes TD, Nery M, Parisi MC. Risk factors for ulceration and amputation in diabetic foot: study in a cohort of 496 patients. Endocrine. 2013; 44:119–24.
Article47. Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care. 2000; 23:606–11.
Article48. Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer: the Seattle Diabetic Foot Study. Diabetes Care. 1999; 22:1036–42.
Article49. Sohn MW, Budiman-Mak E, Lee TA, Oh E, Stuck RM. Significant J-shaped association between body mass index (BMI) and diabetic foot ulcers. Diabetes Metab Res Rev. 2011; 27:402–9.
Article50. Li X, Xiao T, Wang Y, Gu H, Liu Z, Jiang Y, et al. Incidence, risk factors for amputation among patients with diabetic foot ulcer in a Chinese tertiary hospital. Diabetes Res Clin Pract. 2011; 93:26–30.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention and Education for Diabetic Foot
- Investigating Diabetic Foot Pathophysiology and Amputation Prevention Strategies through Behavioral Modification
- Analysis of the Risk Factors for Lower Extremity Amputation due to Diabetic Foot Complications
- Relationship between clinical course and measures of atherosclerosis in diabetic foot
- Orthotic and Prosthetic Options after Diabetic Foot Amputation