Cancer Res Treat.
2022 Oct;54(4):985-995. 10.4143/crt.2021.857.
Assessment of Anti-tumor Efficacy of Osimertinib in Non-Small Cell Lung Cancer Patients by Liquid Biopsy Using Bronchoalveolar Lavage Fluid, Plasma, or Pleural Effusion
- Affiliations
-
- 1Department of Pulmonary and Critical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2534178
- DOI: http://doi.org/10.4143/crt.2021.857
Abstract
- Purpose
This study was to evaluate anti-tumor efficacy of osimertinib in patients positive for acquired epidermal growth factor receptor (EGFR) T790M mutation in liquid biopsy using plasma, bronchoalveolar lavage fluid (BALF) or bronchial washing fluid (BWF), and pleural effusion.
Materials and Methods
Among patients benefited from previous EGFR‒tyrosine kinase inhibitor treatment followed by treatment failure, patients in whom T790M mutations are detected in at least one of the samples including tumor tissues, BALF/BWF, plasma, and pleural effusion were enrolled. T790M mutation was detected by extracting cell free DNA from liquid biopsy samples, using PANA Mutyper. Objective response rate (ORR) and progression-free survival (PFS) with osimertinib treatment were evaluated.
Results
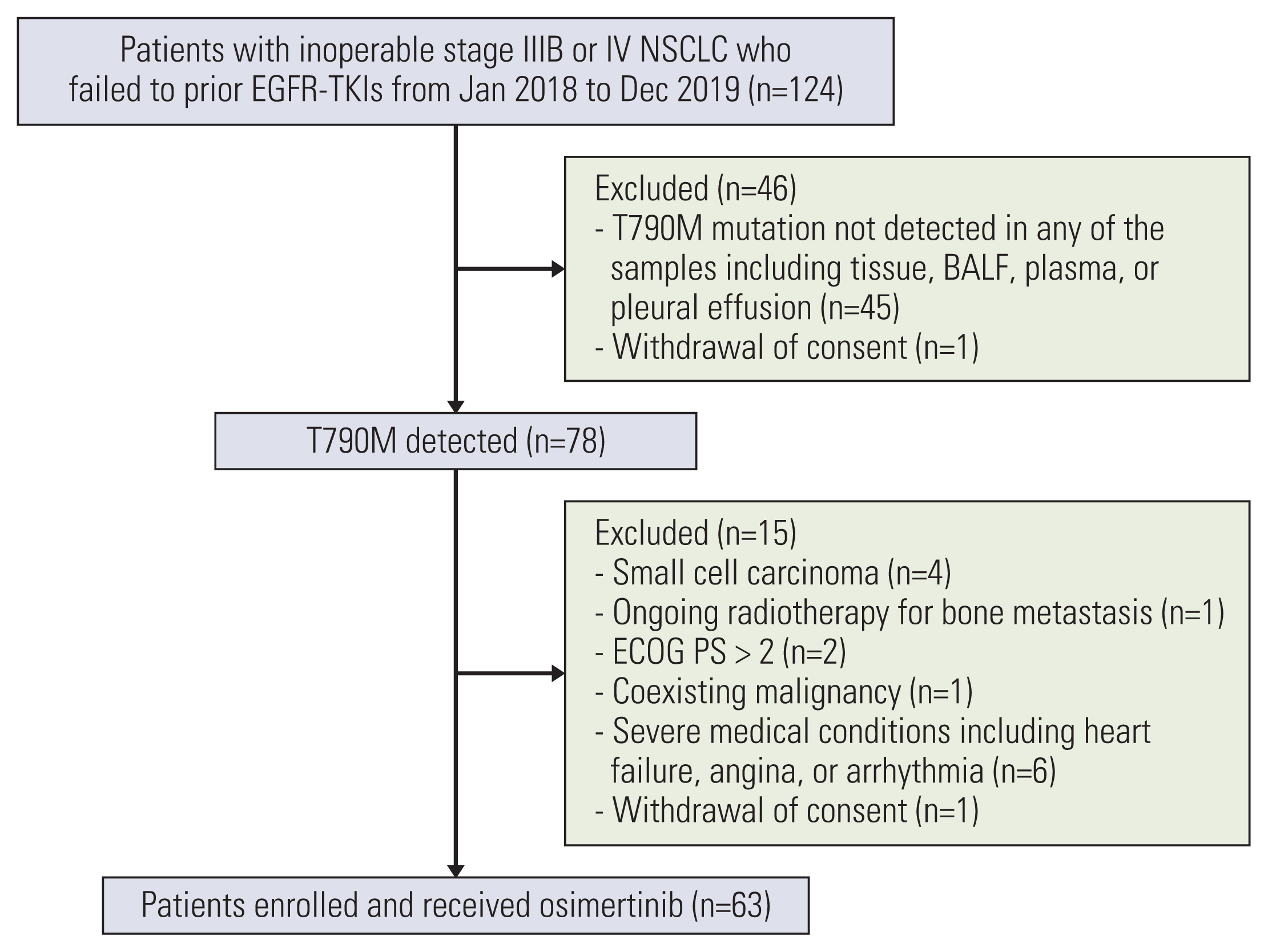
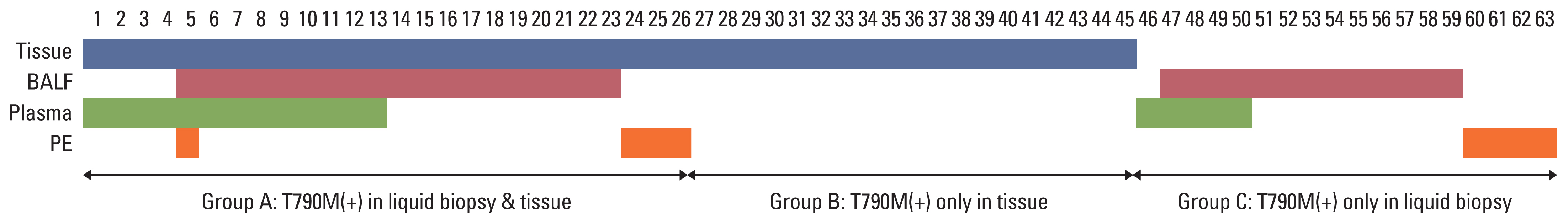
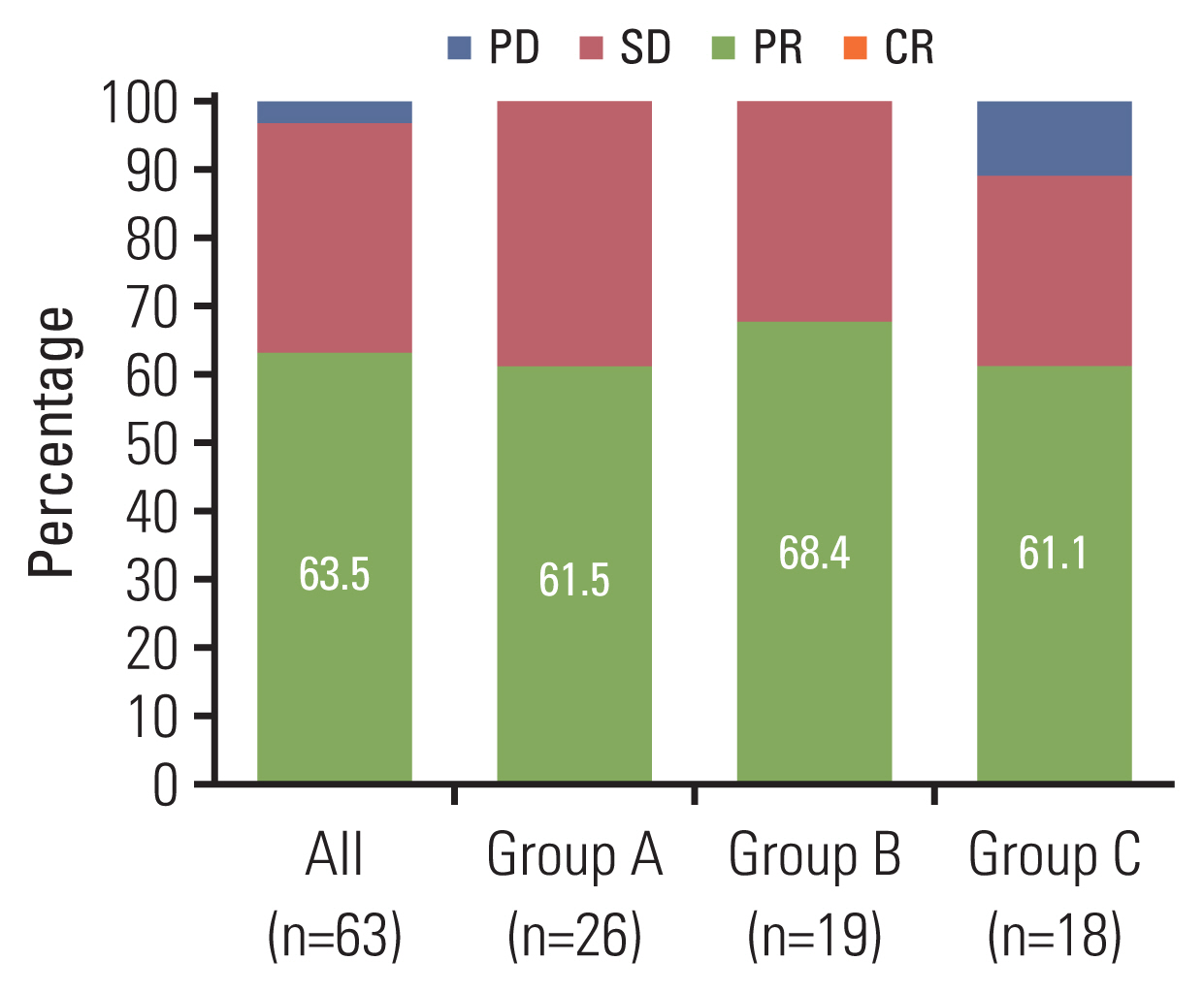
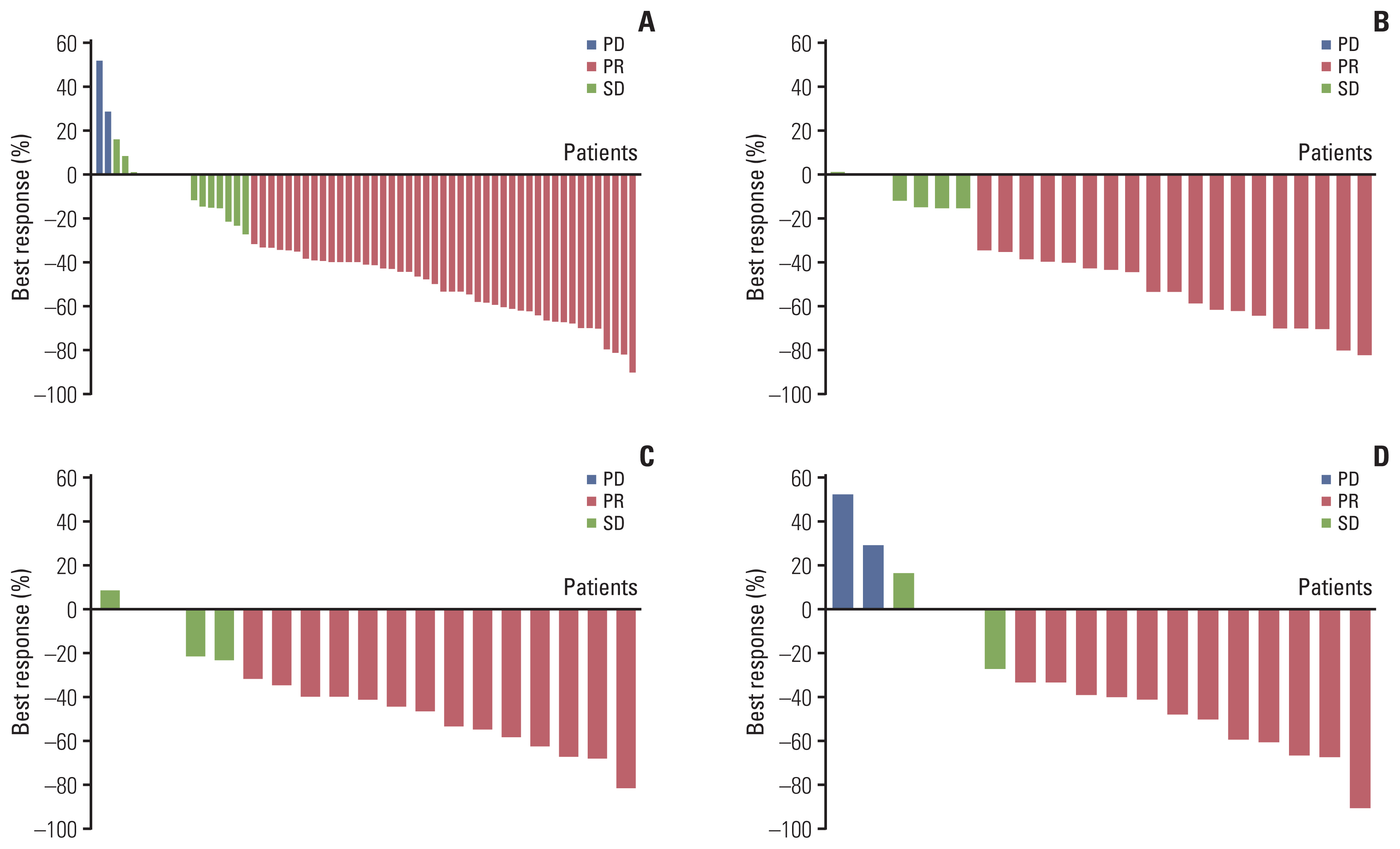
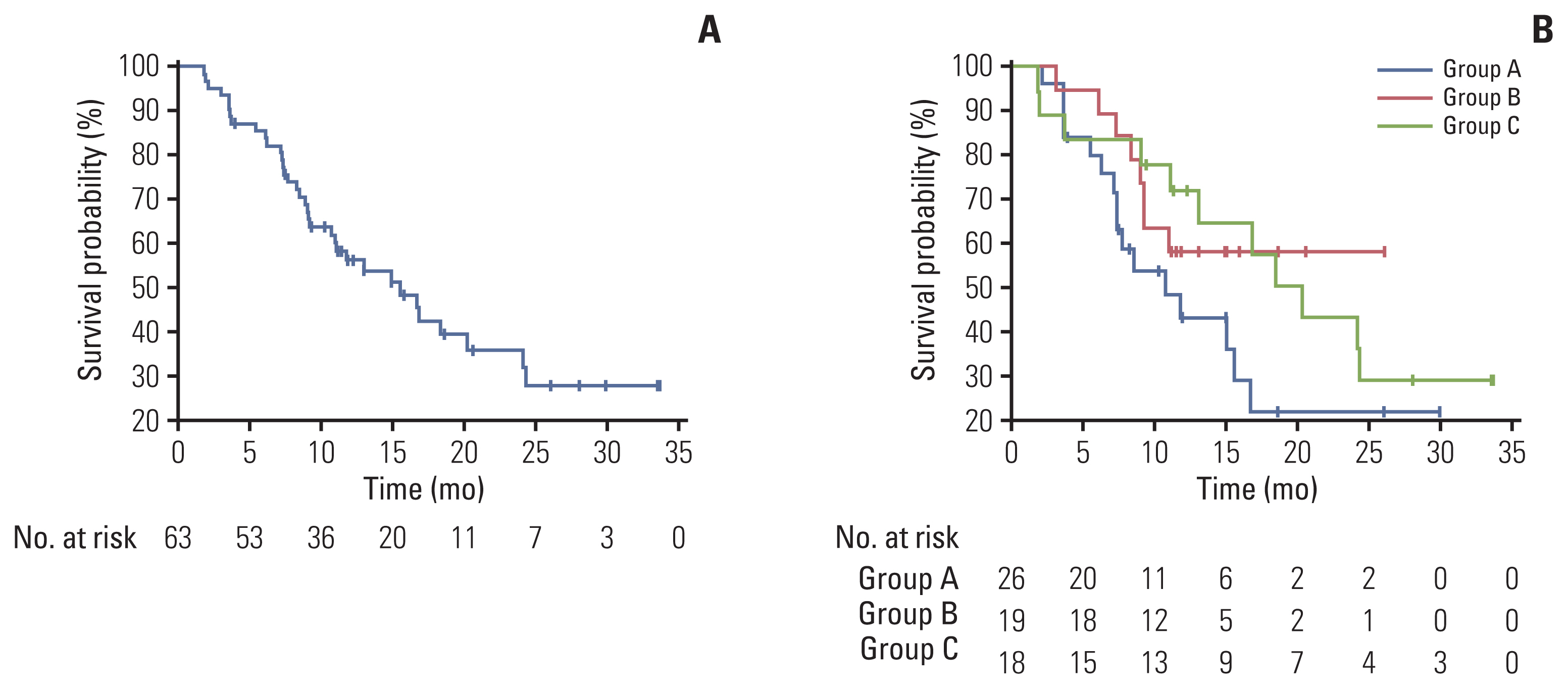
Between January 2018 and December 2019, 63 patients were enrolled and received osimertinib. Mean age was 63 years, and 38 (60.3%) were female. Twenty-six patients had T790M mutation in both liquid and tissue samples (group A), 19 patients had only in tissue biopsy samples (group B), and 18 patients had T790M mutation only in liquid biopsy samples (group C). ORR in overall population was 63.5%, and was 61.5% in group A, 68.4% in group B, and 61.1% in group C, respectively. Median PFS in overall patients was 15.6 months (95% confidence interval, 10.7 to 24.2). There was no significant difference in ORR or PFS between groups.
Conclusion
Osimertinib showed favorable efficacy in lung cancer patients with acquired resistance to prior EGFR-TKI therapies, who screened positive for harboring T790M mutation detected from cell free DNA extracted from plasma, BALF/BWF, and pleural effusion.
Figure
Reference
-
References
1. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009; 361:947–57.2. Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009; 361:958–67.3. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017; 376:629–40.4. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018; 378:113–25.5. FDA approves osimertinib for first-line treatment of metastatic NSCLC with most common EGFR mutations [Internet]. Silver Spring MD: U.S Food and Drug Administration;2018. [cited 2020 Nov 20]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-osimertinib-first-line-treatment-metastatic-nsclc-most-common-egfr-mutations .6. List of reimbursable drugs [Internet]. Wonju: Health Insurance Review and Assessment Service;2018. [cited 2020 Nov 20]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA030014050000 .7. Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016; 5:288–300.8. Denis MG, Lafourcade MP, Le Garff G, Dayen C, Falchero L, Thomas P, et al. Circulating free tumor-derived DNA to detect EGFR mutations in patients with advanced NSCLC: French subset analysis of the ASSESS study. J Thorac Dis. 2019; 11:1370–8.9. Qiu M, Wang J, Xu Y, Ding X, Li M, Jiang F, et al. Circulating tumor DNA is effective for the detection of EGFR mutation in non-small cell lung cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2015; 24:206–12.10. Ryu JS, Lim JH, Lee MK, Lee SJ, Kim HJ, Kim MJ, et al. Feasibility of bronchial washing fluid-based approach to early-stage lung cancer diagnosis. Oncologist. 2019; 24:e603–6.11. Lee SH, Kim EY, Kim T, Chang YS. Compared to plasma, bronchial washing fluid shows higher diagnostic yields for detecting EGFR-TKI sensitizing mutations by ddPCR in lung cancer. Respir Res. 2020; 21:142.12. Rantakokko-Jalava K, Jalava J. Optimal DNA isolation method for detection of bacteria in clinical specimens by broad-range PCR. J Clin Microbiol. 2002; 40:4211–7.13. Han HS, Lim SN, An JY, Lee KM, Choe KH, Lee KH, et al. Detection of EGFR mutation status in lung adenocarcinoma specimens with different proportions of tumor cells using two methods of differential sensitivity. J Thorac Oncol. 2012; 7:355–64.14. Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015; 372:1689–99.15. Chouaid C, Dujon C, Do P, Monnet I, Madroszyk A, Le Caer H, et al. Feasibility and clinical impact of re-biopsy in advanced non small-cell lung cancer: a prospective multicenter study in a real-world setting (GFPC study 12-01). Lung Cancer. 2014; 86:170–3.16. Nosaki K, Satouchi M, Kurata T, Yoshida T, Okamoto I, Katakami N, et al. Re-biopsy status among non-small cell lung cancer patients in Japan: a retrospective study. Lung Cancer. 2016; 101:1–8.17. Kawamura T, Kenmotsu H, Taira T, Omori S, Nakashima K, Wakuda K, et al. Rebiopsy for patients with non-small-cell lung cancer after epidermal growth factor receptor-tyrosine kinase inhibitor failure. Cancer Sci. 2016; 107:1001–5.18. Molina-Vila MA. Liquid biopsy in lung cancer: present and future. Transl Lung Cancer Res. 2016; 5:452–4.19. Jenkins S, Yang JC, Ramalingam SS, Yu K, Patel S, Weston S, et al. Plasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2017; 12:1061–70.20. Karlovich C, Goldman JW, Sun JM, Mann E, Sequist LV, Konopa K, et al. Assessment of EGFR mutation status in matched plasma and tumor tissue of NSCLC patients from a phase I study of rociletinib (CO-1686). Clin Cancer Res. 2016; 22:2386–95.21. Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol. 2016; 34:3375–82.22. Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O’Connell A, Feeney N, et al. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2016; 2:1014–22.23. Helman E, Nguyen M, Karlovich CA, Despain D, Choquette AK, Spira AI, et al. Cell-free DNA next-generation sequencing prediction of response and resistance to third-generation EGFR inhibitor. Clin Lung Cancer. 2018; 19:518–30.24. Papadimitrakopoulou VA, Han JY, Ahn MJ, Ramalingam SS, Delmonte A, Hsia TC, et al. Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: Osimertinib versus platinum-pemetrexed for T790M mutation-positive advanced non-small cell lung cancer. Cancer. 2020; 126:373–80.25. Park CK, Cho HJ, Choi YD, Oh IJ, Kim YC. A phase II trial of osimertinib in the second-line treatment of non-small cell lung cancer with the EGFR T790M mutation, detected from circulating tumor DNA: LiquidLung-O-Cohort 2. Cancer Res Treat. 2019; 51:777–87.26. Poletti V, Poletti G, Murer B, Saragoni L, Chilosi M. Bronchoalveolar lavage in malignancy. Semin Respir Crit Care Med. 2007; 28:534–45.27. Park S, Hur JY, Lee KY, Lee JC, Rho JK, Shin SH, et al. Assessment of EGFR mutation status using cell-free DNA from bronchoalveolar lavage fluid. Clin Chem Lab Med. 2017; 55:1489–95.28. Hur JY, Lee JS, Kim IA, Kim HJ, Kim WS, Lee KY. Extracellular vesicle-based EGFR genotyping in bronchoalveolar lavage fluid from treatment-naive non-small cell lung cancer patients. Transl Lung Cancer Res. 2019; 8:1051–60.29. Kiura K, Yoh K, Katakami N, Nogami N, Kasahara K, Takahashi T, et al. Osimertinib in patients with epidermal growth factor receptor T790M advanced non-small cell lung cancer selected using cytology samples. Cancer Sci. 2018; 109:1177–84.30. Lee JS, Hur JY, Kim IA, Kim HJ, Choi CM, Lee JC, et al. Liquid biopsy using the supernatant of a pleural effusion for EGFR genotyping in pulmonary adenocarcinoma patients: a comparison between cell-free DNA and extracellular vesicle-derived DNA. BMC Cancer. 2018; 18:1236.31. Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Clin Oncol. 2017; 35:1288–96.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Adenosine Deaminase and CYFRA 21-1 in the Differential Diagnosis of Tuberculous and Malignant Pleural Effusion
- Acute Eosinophilic Pneumonia: A Case Report
- The bronchoalveolar lavage fluid cell analysis with normal lung and unaffected side lung of patients with minor symptoms or radiologic abnormalities
- Morphometric Analysis for Pulmonary Small Cell Carcinoma Using Image Analysis
- Changes of the cellularities in the bronchoalveolar lavage fluid of the experimental silicosis