Nutr Res Pract.
2022 Oct;16(5):589-603. 10.4162/nrp.2022.16.5.589.
Effect of blended protein nutritional support on reducing burn-induced inflammation and organ injury
- Affiliations
-
- 1China-Canada Joint Lab of Food Nutrition and Health (Beijing), Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University, Beijing 100048, China
- 2Institute of Food and Nutrition Development, Ministry of Agriculture and Rural Affairs, Beijing 100081, China
- 3Burn Institute, the Fourth Medical Center of PLA General Hospital, Beijing 100048, China
- KMID: 2534120
- DOI: http://doi.org/10.4162/nrp.2022.16.5.589
Abstract
- BACKGROUND/OBJECTIVES
Previous studies have reported that protein supplementation contributes to the attenuation of inflammation. Serious trauma such as burn injury usually results in the excessive release of inflammatory factors and organs dysfunction. However, a few reports continued to focus on the function of protein ingestion in regulating burninduced inflammation and organ dysfunction.
MATERIALS/METHODS
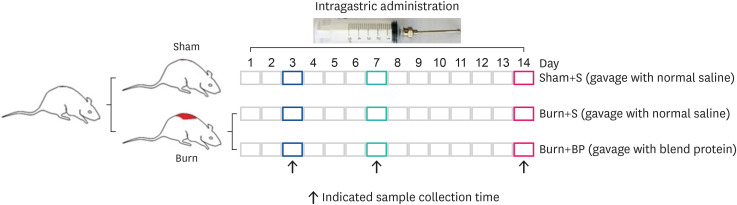
This study established the rat model of 30% total body surface area burn injury, and evaluated the function of blended protein (mixture of whey and soybean proteins). Blood routine examination, inflammatory factors, blood biochemistry, and immunohistochemical assays were employed to analyze the samples from different treatment groups.
RESULTS
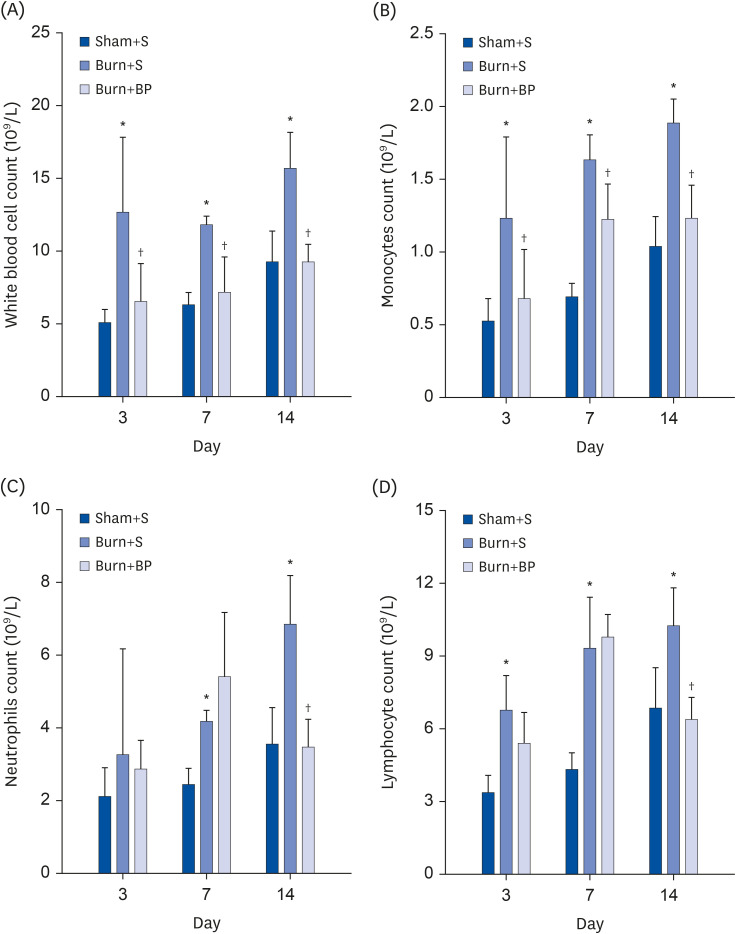
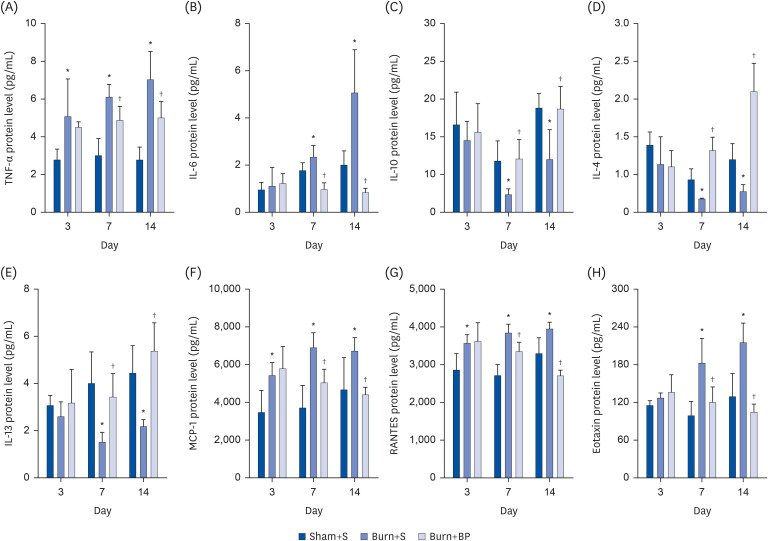
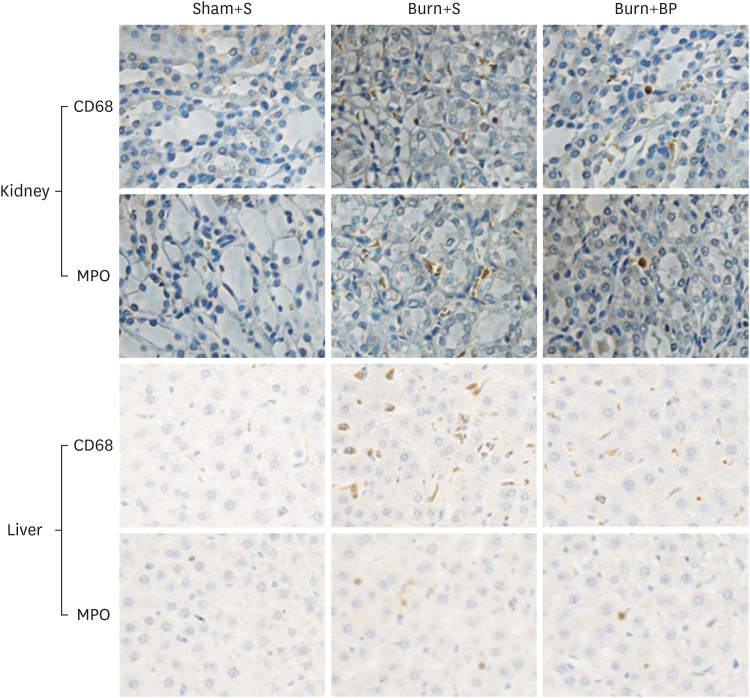
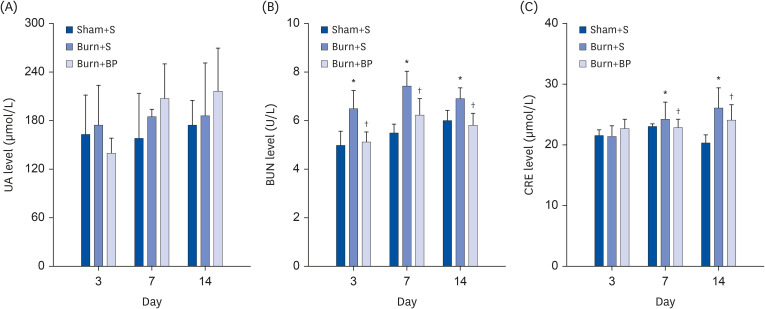
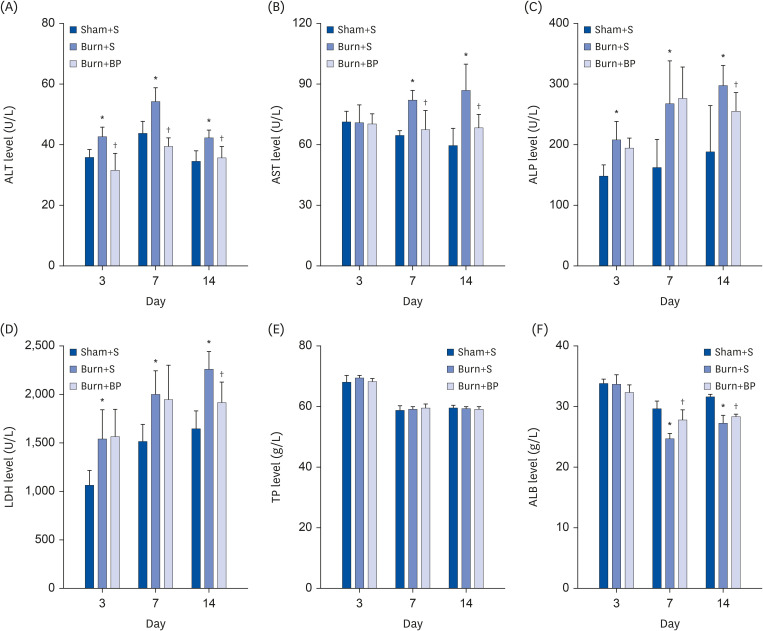
Our results indicated a decrease in the numbers of white blood cells, monocytes, and neutrophils in the burn injury group administered with the blended protein nutritional support (Burn+BP), as compared to the burn injury group administered normal saline supplementation (Burn+S). Expressions of the pro-inflammatory factors (tumor necrosis factor-α and interleukin-6 [IL-6]) and chemokines (macrophage chemoattractant protein-1, regulated upon activation normal T cell expressed and secreted factor, and C-C motif chemokine 11) were dramatically decreased, whereas anti-inflammatory factors (IL-4, IL-10, and IL-13) were significantly increased in the Burn+BP group. Kidney function related markers blood urea nitrogen and serum creatinine, and the liver function related markers alanine transaminase, aspartate aminotransferase, alkaline phosphatase, and lactate dehydrogenase were remarkably reduced, whereas albumin levels were elevated in the Burn+BP group as compared to levels obtained in the Burn+S group. Furthermore, inflammatory cells infiltration of the kidney and liver was also attenuated after burn injury administered with blended protein supplementation.
CONCLUSIONS
In summary, nutritional support with blended proteins dramatically attenuates the burn-induced inflammatory reaction and protects organ functions. We believe this is a new insight into a potential therapeutic strategy for nutritional support of burn patients.
Keyword
Figure
Reference
-
1. Yu Y, Gaine GK, Zhou L, Zhang J, Wang J, Sun B. The classical and potential novel healthy functions of rice bran protein and its hydrolysates. Crit Rev Food Sci Nutr. 2021.
Article2. Liberman K, Njemini R, Luiking Y, Forti LN, Verlaan S, Bauer JM, Memelink R, Brandt K, Donini LM, Maggio M, et al. Thirteen weeks of supplementation of vitamin D and leucine-enriched whey protein nutritional supplement attenuates chronic low-grade inflammation in sarcopenic older adults: the PROVIDE study. Aging Clin Exp Res. 2019; 31:845–854. PMID: 31049877.
Article3. Shin JE, Park SJ, Ahn SI, Choung SY. Soluble whey protein hydrolysate ameliorates muscle atrophy induced by immobilization via regulating the PI3K/Akt pathway in C57BL/6 mice. Nutrients. 2020; 12:3362.
Article4. Zhang J, Yu Y, Wang J. Protein nutritional support: the classical and potential new mechanisms in the prevention and therapy of sarcopenia. J Agric Food Chem. 2020; 68:4098–4108. PMID: 32202113.
Article5. Yu Y, Zhang J, Wang J, Sun B. MicroRNAs: the novel mediators for nutrient-modulating biological functions. Trends Food Sci Technol. 2021; 114:167–175.
Article6. Yu Y, Zhou L, Li X, Liu J, Li H, Gong L, Zhang J, Wang J, Sun B. The progress of nomenclature, structure, metabolism, and bioactivities of oat novel phytochemical: avenanthramides. J Agric Food Chem. 2022; 70:446–457. PMID: 34994561.
Article7. Bitzer ZT, Wopperer AL, Chrisfield BJ, Tao L, Cooper TK, Vanamala J, Elias RJ, Hayes JE, Lambert JD. Soy protein concentrate mitigates markers of colonic inflammation and loss of gut barrier function in vitro and in vivo . J Nutr Biochem. 2017; 40:201–208. PMID: 27951472.
Article8. Da Silva MS, Bigo C, Barbier O, Rudkowska I. Whey protein hydrolysate and branched-chain amino acids downregulate inflammation-related genes in vascular endothelial cells. Nutr Res. 2017; 38:43–51. PMID: 28381353.
Article9. Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Cope MB, Mukherjea R, Jennings K, Volpi E, et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol (1985). 2014; 116:1353–1364. PMID: 24699854.
Article10. Ren G, Yi S, Zhang H, Wang J. Ingestion of soy-whey blended protein augments sports performance and ameliorates exercise-induced fatigue in a rat exercise model. Food Funct. 2017; 8:670–679. PMID: 28121323.
Article11. Liu J, Klebach M, Visser M, Hofman Z. Amino acid availability of a dairy and vegetable protein blend compared to single casein, whey, soy, and pea proteins: a double-blind, cross-over trial. Nutrients. 2019; 11:2613.
Article12. Butteiger DN, Cope M, Liu P, Mukherjea R, Volpi E, Rasmussen BB, Krul ES. A soy, whey and caseinate blend extends postprandial skeletal muscle protein synthesis in rats. Clin Nutr. 2013; 32:585–591. PMID: 23127543.
Article13. Ren G, Zhang J, Li M, Tang Z, Yang Z, Cheng G, Wang J. Gut microbiota composition influences outcomes of skeletal muscle nutritional intervention via blended protein supplementation in posttransplant patients with hematological malignancies. Clin Nutr. 2021; 40:94–102. PMID: 32402683.
Article14. Yu Y, Chai J, Zhang H, Chu W, Liu L, Ma L, Duan H, Li B, Li D. miR-194 Promotes burn-induced hyperglycemia via attenuating IGF-IR expression. Shock. 2014; 42:578–584. PMID: 25186839.
Article15. Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome derived from human umbilical cord mesenchymal stem cell mediates MiR-181c attenuating burn-induced excessive inflammation. EBioMedicine. 2016; 8:72–82. PMID: 27428420.
Article16. Liu L, Yu Y, Hou Y, Chai J, Duan H, Chu W, Zhang H, Hu Q, Du J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014; 9:e88348. PMID: 24586314.
Article17. Zhao F, Liu W, Yu YH, Liu XQ, Yin HN, Liu LY, Yi GF. Effect of small molecular weight soybean protein-derived peptide supplementation on attenuating burn injury-induced inflammation and accelerating wound healing in a rat model. RSC Advances. 2019; 9:1247–1259. PMID: 35518054.
Article18. Yu Y, Yang L, Han S, Wu Y, Liu L, Chang Y, Wang X, Chai J. MIR-190B alleviates cell autophagy and burn-induced skeletal muscle wasting via modulating PHLPP1/Akt/FoxO3A signaling pathway. Shock. 2019; 52:513–521. PMID: 30407372.
Article19. Zhao F, Yu Y, Liu W, Zhang J, Liu X, Liu L, Yin H. Small Molecular weight soybean protein-derived peptides nutriment attenuates rat burn injury-induced muscle atrophy by modulation of ubiquitin-proteasome system and autophagy signaling pathway. J Agric Food Chem. 2018; 66:2724–2734. PMID: 29493231.
Article20. Pecht T, Gutman-Tirosh A, Bashan N, Rudich A. Peripheral blood leucocyte subclasses as potential biomarkers of adipose tissue inflammation and obesity subphenotypes in humans. Obes Rev. 2014; 15:322–337. PMID: 24251825.
Article21. Rondanelli M, Klersy C, Terracol G, Talluri J, Maugeri R, Guido D, Faliva MA, Solerte BS, Fioravanti M, Lukaski H, et al. Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr. 2016; 103:830–840. PMID: 26864356.
Article22. Sakuma M, Khan MA, Yasuhara S, Martyn JA, Palaniyar N. Mechanism of pulmonary immunosuppression: extrapulmonary burn injury suppresses bacterial endotoxin-induced pulmonary neutrophil recruitment and neutrophil extracellular trap (NET) formation. FASEB J. 2019; 33:13602–13616. PMID: 31577450.
Article23. Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019; 15:9–17. PMID: 30341437.
Article24. Haller H, Bertram A, Nadrowitz F, Menne J. Monocyte chemoattractant protein-1 and the kidney. Curr Opin Nephrol Hypertens. 2016; 25:42–49. PMID: 26625862.
Article25. Fichna M, Żurawek M, Budny B, Komarowska H, Niechciał E, Fichna P, Ruchała M. Elevated serum RANTES chemokine levels in autoimmune Addison disease. Pol Arch Intern Med. 2018; 128:216–221. PMID: 29498364.26. Rankin SM, Conroy DM, Williams TJ. Eotaxin and eosinophil recruitment: implications for human disease. Mol Med Today. 2000; 6:20–27. PMID: 10637571.
Article27. Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG, Yarmush ML. Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant. 2010; 19:823–830. PMID: 20573305.
Article28. Inoue Y, Yu YM, Kurihara T, Vasilyev A, Ibrahim A, Oklu R, Zhao G, Nair AV, Brown D, Fischman AJ, et al. Kidney and liver injuries after major burns in rats are prevented by resolvin D2. Crit Care Med. 2016; 44:e241–e252. PMID: 26509319.
Article29. Kaneko Y, Cho T, Sato Y, Goto K, Yamamoto S, Goto S, Madaio MP, Narita I. Attenuated macrophage infiltration in glomeruli of aged mice resulting in ameliorated kidney injury in nephrotoxic serum nephritis. J Gerontol A Biol Sci Med Sci. 2018; 73:1178–1186. PMID: 29415117.
Article30. Lamas-Paz A, Hao F, Nelson LJ, Vázquez MT, Canals S, Gómez Del Moral M, Martínez-Naves E, Nevzorova YA, Cubero FJ. Alcoholic liver disease: utility of animal models. World J Gastroenterol. 2018; 24:5063–5075. PMID: 30568384.
Article31. Kołodziej U, Maciejczyk M, Niklińska W, Waszkiel D, Żendzian-Piotrowska M, Żukowski P, Zalewska A. Chronic high-protein diet induces oxidative stress and alters the salivary gland function in rats. Arch Oral Biol. 2017; 84:6–12. PMID: 28926744.
Article32. Machín M, Simoyi MF, Blemings KP, Klandorf H. Increased dietary protein elevates plasma uric acid and is associated with decreased oxidative stress in rapidly-growing broilers. Comp Biochem Physiol B Biochem Mol Biol. 2004; 137:383–390. PMID: 15050525.
Article33. Pan J, Shi M, Ma L, Fu P. Mechanistic insights of soluble uric acid-related kidney disease. Curr Med Chem. 2020; 27:5056–5066. PMID: 30526453.
Article34. Ng JW, Cairns SA, O’Boyle CP. Management of the lower gastrointestinal system in burn: a comprehensive review. Burns. 2016; 42:728–737. PMID: 26774605.
Article35. Babajafari S, Hojhabrimanesh A, Sohrabi Z, Ayaz M, Noorafshan A, Akrami A. Comparing isolated soy protein with flaxseed oil vs isolated soy protein with corn oil and wheat flour with corn oil consumption on muscle catabolism, liver function, blood lipid, and sugar in burn patients: a randomized clinical trial. Trials. 2018; 19:308. PMID: 29866187.
Article36. Nelms CL. Optimizing enteral nutrition for growth in pediatric chronic kidney disease (CKD). Front Pediatr. 2018; 6:214. PMID: 30116725.
Article37. McGraw NJ, Krul ES, Grunz-Borgmann E, Parrish AR. Soy-based renoprotection. World J Nephrol. 2016; 5:233–257. PMID: 27152261.
Article38. Li M, Zhao H, Wu J, Wang L, Wang J, Lv K, Liu S, Wang M, Guan W, Liu J, et al. Nobiletin protects against acute liver injury via targeting c-Jun N-terminal kinase (JNK)-induced apoptosis of hepatocytes. J Agric Food Chem. 2020; 68:7112–7120. PMID: 32538091.
Article39. Qiao M, Yang J, Zhu Y, Zhao Y, Hu J. Transcriptomics and proteomics analysis of system-level mechanisms in the liver of apigenin-treated fibrotic rats. Life Sci. 2020; 248:117475. PMID: 32119963.
Article40. Epting CL, McBride ME, Wald EL, Costello JM. Pathophysiology of post-operative low cardiac output syndrome. Curr Vasc Pharmacol. 2016; 14:14–23. PMID: 26463989.
Article41. Clark A, Imran J, Madni T, Wolf SE. Nutrition and metabolism in burn patients. Burns Trauma. 2017; 5:11. PMID: 28428966.
Article42. Klein GL. The role of the musculoskeletal system in post-burn hypermetabolism. Metabolism. 2019; 97:81–86. PMID: 31181216.
Article43. Rousseau AF, Losser MR, Ichai C, Berger MM. ESPEN endorsed recommendations: nutritional therapy in major burns. Clin Nutr. 2013; 32:497–502. PMID: 23582468.
Article44. Moore FA, Phillips SM, McClain CJ, Patel JJ, Martindale RG. Nutrition support for persistent inflammation, immunosuppression, and catabolism syndrome. Nutr Clin Pract. 2017; 32:121S–127S. PMID: 28166447.
Article45. Lami FH, Al Naser RK. Epidemiological characteristics of burn injuries in Iraq: a burn hospital-based study. Burns. 2019; 45:479–483. PMID: 30600127.
Article